GPR40 agonist ameliorates liver X receptor-induced lipid accumulation in liver by activating AMPK pathway
- PMID: 27121981
- PMCID: PMC4848522
- DOI: 10.1038/srep25237
GPR40 agonist ameliorates liver X receptor-induced lipid accumulation in liver by activating AMPK pathway
Abstract
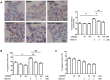
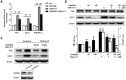
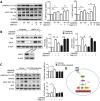
Hepatic steatosis is strongly linked to insulin resistance and type 2 diabetes. GPR40 is a G protein-coupled receptor mediating free fatty acid-induced insulin secretion and thus plays a beneficial role in the improvement of diabetes. However, the impact of GPR40 agonist on hepatic steatosis still remains to be elucidated. In the present study, we found that activation of GPR40 by its agonist GW9508 attenuated Liver X receptor (LXR)-induced hepatic lipid accumulation. Activation of LXR in the livers of C57BL/6 mice fed a high-cholesterol diet and in HepG2 cells stimulated by chemical agonist caused increased expression of its target lipogenic genes and subsequent lipid accumulation. All these effects of LXR were dramatically downregulated after GW9508 supplementation. Moreover, GPR40 activation was accompanied by upregulation of AMPK pathway, whereas the inhibitive effect of GPR40 on the lipogenic gene expression was largely abrogated by AMPK knockdown. Taken together, our results demonstrated that GW9508 exerts a beneficial effect to ameliorate LXR-induced hepatic steatosis through regulation of AMPK signaling pathway.
Figures


References
-
- Sanyal A. J. Mechanisms of disease: pathogenesis of nonalcoholic fatty liver disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2, 46–53 (2005). - PubMed
-
- Peet D. J. et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα. Cell 93, 693–704 (1998). - PubMed
-
- Repa J. et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289, 1524–1529 (2000). - PubMed
-
- Repa J. J. & Mangelsdorf D. J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 16, 459–481 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

