Oligodendrogliopathy in Multiple Sclerosis: Low Glycolytic Metabolic Rate Promotes Oligodendrocyte Survival
- PMID: 27122029
- PMCID: PMC6601725
- DOI: 10.1523/JNEUROSCI.4077-15.2016
Oligodendrogliopathy in Multiple Sclerosis: Low Glycolytic Metabolic Rate Promotes Oligodendrocyte Survival
Abstract
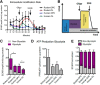
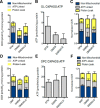
Multiple sclerosis (MS) lesions feature demyelination with limited remyelination. A distinct injury phenotype of MS lesions features dying back of oligodendrocyte (OL) terminal processes, a response that destabilizes myelin/axon interactions. This oligodendrogliopathy has been linked with local metabolic stress, similar to the penumbra of ischemic/hypoxic states. Here, we developed an in vitro oligodendrogliopathy model using human CNS-derived OLs and related this injury response to their distinct bioenergetic properties. We determined the energy utilization properties of adult human surgically derived OLs cultured under either optimal or metabolic stress conditions, deprivation of growth factors, and glucose and/or hypoxia using a Seahorse extracellular flux analyzer. Baseline studies were also performed on OL progenitor cells derived from the same tissue and postnatal rat-derived cells. Under basal conditions, adult human OLs were less metabolically active than their progenitors and both were less active than the rat cells. Human OLs and progenitors both used aerobic glycolysis for the majority of ATP production, a process that contributes to protein and lipid production necessary for myelin biosynthesis. Under stress conditions that induce significant process retraction with only marginal cell death, human OLs exhibited a significant reduction in overall energy utilization, particularly in glycolytic ATP production. The stress-induced reduction of glycolytic ATP production by the human OLs would exacerbate myelin process withdrawal while favoring cell survival, providing a potential basis for the oligodendrogliopathy observed in MS. The glycolytic pathway is a potential therapeutic target to promote myelin maintenance and enhance repair in MS.
Significance statement: The neurologic deficits that characterize multiple sclerosis (MS) reflect disruption of myelin (demyelination) within the CNS and failure of repair (remyelination). We define distinct energy utilization properties of human adult brain-derived oligodendrocytes and oligodendrocyte progenitor cells under conditions of metabolic stress that model the initial relapsing and subsequent progressive phases of MS. The observed changes in energy utilization affect both cell survival and myelination capacity. These processes may be amenable to therapeutic interventions to limit the extent of cumulative tissue injury and to promote repair in MS.
Keywords: aerobic glycolysis; metabolic stress; multiple sclerosis; oligodendrocytes; oligodendrogliopathy.
Copyright © 2016 the authors 0270-6474/16/364698-10$15.00/0.
Figures






References
-
- Aboul-Enein F, Rauschka H, Kornek B, Stadelmann C, Stefferl A, Brück W, Lucchinetti C, Schmidbauer M, Jellinger K, Lassmann H. Preferential loss of myelin-associated glycoprotein reflects hypoxia-like white matter damage in stroke and inflammatory brain diseases. J Neuropathol Exp Neurol. 2003;62:25–33. doi: 10.1093/jnen/62.1.25. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
