An initial phase of JNK activation inhibits cell death early in the endoplasmic reticulum stress response
- PMID: 27122189
- PMCID: PMC5172423
- DOI: 10.1242/jcs.179127
An initial phase of JNK activation inhibits cell death early in the endoplasmic reticulum stress response
Abstract
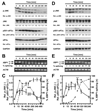
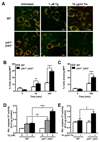
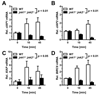
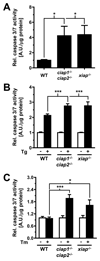
Accumulation of unfolded proteins in the endoplasmic reticulum (ER) activates the unfolded protein response (UPR). In mammalian cells, UPR signals generated by several ER-membrane-resident proteins, including the bifunctional protein kinase endoribonuclease IRE1α, control cell survival and the decision to execute apoptosis. Processing of XBP1 mRNA by the RNase domain of IRE1α promotes survival of ER stress, whereas activation of the mitogen-activated protein kinase JNK family by IRE1α late in the ER stress response promotes apoptosis. Here, we show that activation of JNK in the ER stress response precedes activation of XBP1. This activation of JNK is dependent on IRE1α and TRAF2 and coincides with JNK-dependent induction of expression of several antiapoptotic genes, including cIap1 (also known as Birc2), cIap2 (also known as Birc3), Xiap and Birc6 ER-stressed Jnk1(-/-) Jnk2(-/-) (Mapk8(-/-) Mapk9(-/-)) mouse embryonic fibroblasts (MEFs) display more pronounced mitochondrial permeability transition and increased caspase 3/7 activity compared to wild-type MEFs. Caspase 3/7 activity is also elevated in ER-stressed cIap1(-/-) cIap2(-/-) and Xiap(-/-) MEFs. These observations suggest that JNK-dependent transcriptional induction of several inhibitors of apoptosis contributes to inhibiting apoptosis early in the ER stress response.
Keywords: Apoptosis; Endoplasmic reticulum; IRE1; JNK; Stress response; Unfolded protein response.
© 2016. Published by The Company of Biologists Ltd.
Figures


References
-
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–73. - PubMed
-
- Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, Miller SC, Webster C. Plasticity of the differentiated state. Science. 1985;230:758–66. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

