Copper Exposure Perturbs Brain Inflammatory Responses and Impairs Clearance of Amyloid-Beta
- PMID: 27122238
- PMCID: PMC4922545
- DOI: 10.1093/toxsci/kfw081
Copper Exposure Perturbs Brain Inflammatory Responses and Impairs Clearance of Amyloid-Beta
Abstract
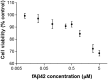
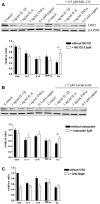
Copper promotes a toxic buildup of amyloid-beta (Aβ) and neurofibrillary tangle pathology in the brain, and its exposure may increase the risk for Alzheimer's disease (AD). However, underlying molecular mechanisms by which copper triggers such pathological changes remain largely unknown. We hypothesized that the copper exposure perturbs brain inflammatory responses, leading to impairment of Aβ clearance from the brain parenchyma. Here, we investigated whether copper attenuated Aβ clearance by microglial phagocytosis or by low-density lipoprotein-related receptor protein-1 (LRP1) dependent transcytosis in both in vitro and in vivo When murine monocyte BV2 cells were exposed to copper, their phagocytic activation induced by fibrillar Aβ or LPS was significantly reduced, while the secretion of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-6, were increased. Interestingly, not only copper itself but also IL-1β, IL-6, or TNF-α were capable of markedly reducing the expression of LRP1 in human microvascular endothelial cells (MVECs) in a concentration-dependent manner. While copper-mediated downregulation of LRP1 was proteasome-dependent, the cytokine-induced loss of LRP1 was proteasome- or lysosome-independent. In the mouse model, copper exposure also significantly elevated neuroinflammation and downregulated LRP1 in the brain, consistent with our in vitro results. Taken together, our findings support the pathological impact of copper on inflammatory responses and Aβ clearance in the brain, which could serve as key mechanisms to explain, in part, the copper exposure as an environmental risk factor for AD.
Keywords: Alzheimer’s disease; LRP1; copper; cytokines; inflammation; microglia; phagocytosis.
© The Author 2016. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


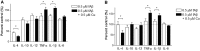


References
-
- Bayer T. A., Schafer S., Simons A., Kemmling A., Kamer T., Tepest R., Eckert A., Schussel K., Eikenberg O., Sturchler-Pierrat C., et al. (2003). Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice. Proc. Natl. Acad. Sci. USA 100, 14187–14192. - PMC - PubMed
-
- Chakrabarty P., Jansen-West K., Beccard A., Ceballos-Diaz C., Levites Y., Verbeeck C., Zubair A. C., Dickson D., Golde T. E., Das P. (2010). Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. Faseb J. 24, 548–559. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

