Dynamic myosin activation promotes collective morphology and migration by locally balancing oppositional forces from surrounding tissue
- PMID: 27122602
- PMCID: PMC4907723
- DOI: 10.1091/mbc.E15-10-0744
Dynamic myosin activation promotes collective morphology and migration by locally balancing oppositional forces from surrounding tissue
Abstract
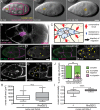
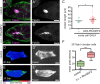
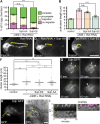
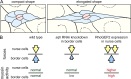
Migrating cells need to overcome physical constraints from the local microenvironment to navigate their way through tissues. Cells that move collectively have the additional challenge of negotiating complex environments in vivo while maintaining cohesion of the group as a whole. The mechanisms by which collectives maintain a migratory morphology while resisting physical constraints from the surrounding tissue are poorly understood. Drosophila border cells represent a genetic model of collective migration within a cell-dense tissue. Border cells move as a cohesive group of 6-10 cells, traversing a network of large germ line-derived nurse cells within the ovary. Here we show that the border cell cluster is compact and round throughout their entire migration, a shape that is maintained despite the mechanical pressure imposed by the surrounding nurse cells. Nonmuscle myosin II (Myo-II) activity at the cluster periphery becomes elevated in response to increased constriction by nurse cells. Furthermore, the distinctive border cell collective morphology requires highly dynamic and localized enrichment of Myo-II. Thus, activated Myo-II promotes cortical tension at the outer edge of the migrating border cell cluster to resist compressive forces from nurse cells. We propose that dynamic actomyosin tension at the periphery of collectives facilitates their movement through restrictive tissues.
© 2016 Aranjuez et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


References
-
- Alexander S, Weigelin B, Winkler F, Friedl P. Preclinical intravital microscopy of the tumour-stroma interface: invasion, metastasis, and therapy response. Curr Opin Cell Biol. 2013;25:659–671. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

