Andrographolide, a Novel NF-κB Inhibitor, Inhibits Vascular Smooth Muscle Cell Proliferation and Cerebral Endothelial Cell Inflammation
- PMID: 27122804
- PMCID: PMC4804993
Andrographolide, a Novel NF-κB Inhibitor, Inhibits Vascular Smooth Muscle Cell Proliferation and Cerebral Endothelial Cell Inflammation
Abstract
Background: Aberrant vascular smooth muscle cell (VSMC) proliferation and cerebral endothelial cell (CEC) dysfunction contribute significantly in the pathogenesis of cardiovascular diseases. Therefore, inhibition of these cellular events would be by candidate agents for treating these diseases. In the present study, the mechanism of anti-proliferative and anti-inflammatory effects of andrographolides, a novel nuclear factor-κB inhibitor, was investigated in VSMC and CEC cells.
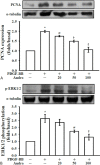
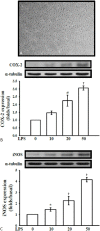
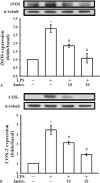
Methods: VSMCs and CECs were isolated from rat artery and mouse brain, respectively, and cultured before experimentation. The effect of andro on platelet-derived growth factor-BB (PDGF-BB) induced VSMC cell proliferation was evaluated by cell number, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The expression of extracellular signal regulated kinase 1/2 (ERK1/2), proliferating cell nuclear antigen (PCNA), and the effects on lipopolysaccharide (LPS)-induced inducible nitric oxide synthase (iNOS) and, cyclooxygenase-2 (COX2) were detected by Western blotting.
Results: Andro significantly inhibited PDGF-BB (10 ng/ml) induced cell proliferation in a concentration (20-100 μM) dependent manner, which may be due to reducing the expression of ERK1/2, and by inhibiting the expression of PCNA. Andro also remarkably diminished LPS-induced iNOS and COX2 expression.
Conclusions: The results of this study suggested that the effects of andro against VSMCs proliferation and CECs dysfunction may represent a promising approach for treatment of vascular diseases.
Key words: Andrographolide; CECs; COX2/iNOS; ERK/PCNA; LPS; PDGF-BB; VSMCs.
Figures

References
-
- Sachinidis A, Locher R, Vetter W, et al. Different effects of platelet-derived growth factor isoforms on rat vascular smooth muscle cells. J Biol Chem. 1990;265:10238–10243. - PubMed
-
- Bornfeldt KE, Raines EW, Graves LM, et al. Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Ann N Y Acad Sci. 1995;766:416–430. - PubMed
-
- Faraci FM, Lentz SR. Hyperhomocysteinemia, oxidative stress, and cerebral vascular dysfunction. Stroke. 2004;35:345–347. - PubMed
-
- Coleman HA, Tare M, Parkington HC. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin Exp Pharmacol Physiol. 2004;31:641–649. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
