Pluripotent Stem Cell Therapy in Ischemic Cardiovascular Disease
- PMID: 27122813
- PMCID: PMC4834953
Pluripotent Stem Cell Therapy in Ischemic Cardiovascular Disease
Abstract
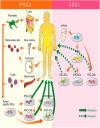
Stem cell therapy has been viewed as a promising therapeutic strategy in ischemic cardiovascular disease for almost a decade. Although many progenitor/stem cells obtained from patients have been investigated, and are alleged to be suitable for autologous transplantation, their therapeutic application has been limited by their inability to yield a sufficient number of stem cells, as well as impaired regeneration capacity from ageing and cardiovascular risk factors. Pluripotent stem cells, such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have the capacity for functional multi-lineage differentiation and properties of self-renewal and immortality, and can generate clinically relevant amounts of stem cells. The regeneration capacity of these cells is not affected by ageing. Patient-specific pluripotent stem cells, iPSCs, can be established by epigenetically reprogramming somatic fibroblasts. iPSCs and iPSC-derived stem cells share similar phenotypes and gene expressions of ESCs and ESC-derived stem cells. Transplantation of pluripotent stem cell-derived endothelial cells, mural cells, cardiomyocytes, or cardiovascular progenitor cells contribute to neovascularization and cardiomyogenesis with better limb perfusion and recovery of myocardial contractility in the preclinical studies. Several strategies have been developed to enhance the efficacy of reprogramming and engrafting, and improve graft survival, proliferation, and electromechanical coupling by tissue engineering. However, the therapeutic application of ESCs and derivatives is limited by ethical concerns. Before wide clinical application of these cells in regeneration therapy occurs, substantial effort should be undertaken to discover the most promising cell type and derivatives, the best protocol regarding cell preparation, reprogramming and differentiation, and the most efficacious methods to avoid adverse effects.
Key words: Embryonic stem cells; Induced pluripotent stem cells; Limb ischemia; Myocardial infarction.
Figures
References
-
- Chao TH, Tseng SY, Li YH, et al. A novel vasculo-angiogenic effect of cilostazol mediated by cross-talk between multiple signalling pathways including the ERK/p38 MAPK signaling transduction cascade. Clin Sci (Lond) 2012;123:147–159. - PubMed
-
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Chao TH, Tseng SY, Chen IC, et al. Cilostazol enhances mobilization and proliferation of endothelial progenitor cells and collateral formation by modifying vasculo-angiogenic biomarkers in peripheral arterial disease. Int J Cardiol. 2014;172:371–374. - PubMed
-
- Yamahara K, Itoh H. Potential use of endothelial progenitor cells for regeneration of the vasculature. Ther Adv Cardiovas. 2009;3:17–27. - PubMed
-
- Sen S, McDonald SP, Coates PT, Bonder CS. Endothelial progenitor cells:novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci (Lond) 2011;120:263–283. - PubMed
Publication types
LinkOut - more resources
Full Text Sources