Netrin-1 Peptide Is a Chemorepellent in Tetrahymena thermophila
- PMID: 27123011
- PMCID: PMC4830718
- DOI: 10.1155/2016/7142868
Netrin-1 Peptide Is a Chemorepellent in Tetrahymena thermophila
Abstract
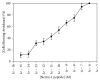
Netrin-1 is a highly conserved, pleiotropic signaling molecule that can serve as a neuronal chemorepellent during vertebrate development. In vertebrates, chemorepellent signaling is mediated through the tyrosine kinase, src-1, and the tyrosine phosphatase, shp-2. Tetrahymena thermophila has been used as a model system for chemorepellent signaling because its avoidance response is easily characterized under a light microscope. Our experiments showed that netrin-1 peptide is a chemorepellent in T. thermophila at micromolar concentrations. T. thermophila adapts to netrin-1 over a time course of about 10 minutes. Netrin-adapted cells still avoid GTP, PACAP-38, and nociceptin, suggesting that netrin does not use the same signaling machinery as any of these other repellents. Avoidance of netrin-1 peptide was effectively eliminated by the addition of the tyrosine kinase inhibitor, genistein, to the assay buffer; however, immunostaining using an anti-phosphotyrosine antibody showed similar fluorescence levels in control and netrin-1 exposed cells, suggesting that tyrosine phosphorylation is not required for signaling to occur. In addition, ELISA indicates that a netrin-like peptide is present in both whole cell extract and secreted protein obtained from Tetrahymena thermophila. Further study will be required in order to fully elucidate the signaling mechanism of netrin-1 peptide in this organism.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

