Bevacizumab treatment for newly diagnosed glioblastoma: Systematic review and meta-analysis of clinical trials
- PMID: 27123291
- PMCID: PMC4840497
- DOI: 10.3892/mco.2016.816
Bevacizumab treatment for newly diagnosed glioblastoma: Systematic review and meta-analysis of clinical trials
Abstract
High-grade glioma is a richly neovascularized brain solid tumor with a poor prognosis. Bevacizumab is a recombinant humanized monoclonal antibody that inhibits vascular endothelial cell proliferation and angiogenesis, which has shown clinical efficacy in recurrent glioblastoma. MEDLINE/PubMed, EMBASE and Web of Science databases were searched for relevant studies that compared bevacizumab plus combined radiotherapy/temozolomide (RT/TMZ) with RT/TMZ alone in newly diagnosed glioblastoma (GBM). Of all the studies identified, three comparative trials were included in the systematic review. All three enrolling trials, including a total of 1,738 patients, investigated bevacizumab or placebo plus combined RT/TMZ treatment in glioblastoma. The result showed no increased overall survival (OS) (pooled hazard ratio (HR), 1.04; 95% confidence interval (CI), 0.84-1.29; P=0.71) but increased progression-free survival (HR, 0.74; 95% CI, 0.62-0.88; P=0.0009). However, the two randomized double-blind placebo-control trials exemplified a high rate of adverse events of the bevacizumab compared with the placebo group while discrepant points were noted in term of quality-of-life outcome. Additionally, bevacizumab plus RT/TMZ did not increase the 6-month survival rate [odd ratios (ORs), 0.65; 95% CI, 0.37-1.13; P=0.13). Overall, addition of bevacizumab to radiotherapy-temozolomide treatment may be an effective therapy strategy for improving progression-free survival. OS and the 6-month survival rate was not prolonged and there was questionable efficacy of bevacizumab on the quality-of-life of glioblastoma patients, thus further clinical trials should be performed.
Keywords: bevacizumab; glioblastoma; overall survival; progression-free survival; radiotherapy; temozolomide.
Figures

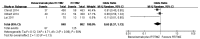
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group: Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
