Comparison of Calcium and Barium Microcapsules as Scaffolds in the Development of Artificial Dermal Papillae
- PMID: 27123456
- PMCID: PMC4829698
- DOI: 10.1155/2016/9128535
Comparison of Calcium and Barium Microcapsules as Scaffolds in the Development of Artificial Dermal Papillae
Abstract
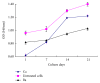
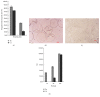
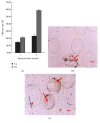
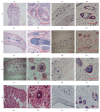
This study aimed to develop and evaluate barium and calcium microcapsules as candidates for scaffolding in artificial dermal papilla. Dermal papilla cells (DPCs) were isolated and cultured by one-step collagenase treatment. The DPC-Ba and DPC-Ca microcapsules were prepared by using a specially designed, high-voltage, electric-field droplet generator. Selected microcapsules were assessed for long-term inductive properties with xenotransplantation into Sprague-Dawley rat ears. Both barium and calcium microcapsules maintained xenogenic dermal papilla cells in an immunoisolated environment and induced the formation of hair follicle structures. Calcium microcapsules showed better biocompatibility, permeability, and cell viability in comparison with barium microcapsules. Before 18 weeks, calcium microcapsules gathered together, with no substantial immune response. After 32 weeks, some microcapsules were near inflammatory cells and wrapped with fiber. A few large hair follicles were found. Control samples showed no marked changes at the implantation site. Barium microcapsules were superior to calcium microcapsules in structural and mechanical stability. The cells encapsulated in hydrogel barium microcapsules exhibited higher short-term viability. This study established a model to culture DPCs in 3D culture conditions. Barium microcapsules may be useful in short-term transplantation study. Calcium microcapsules may provide an effective scaffold for the development of artificial dermal papilla.
Figures



Similar articles
-
Induction of hair follicle regeneration in rat ear by microencapsulated human hair dermal papilla cells.Chin J Traumatol. 2009 Feb;12(1):49-54. Chin J Traumatol. 2009. PMID: 19159517
-
Synergistic effect of PDGF and FGF2 for cell proliferation and hair inductive activity in murine vibrissal dermal papilla in vitro.J Dermatol Sci. 2015 Aug;79(2):110-8. doi: 10.1016/j.jdermsci.2015.04.007. Epub 2015 Apr 27. J Dermatol Sci. 2015. PMID: 25975959
-
Comparison of two types of alginate microcapsules on stability and biocompatibility in vitro and in vivo.Biomed Mater. 2006 Mar;1(1):42-7. doi: 10.1088/1748-6041/1/1/007. Epub 2006 Mar 15. Biomed Mater. 2006. PMID: 18458385
-
Bioengineering the hair follicle: fringe benefits of stem cell technology.Curr Opin Biotechnol. 2005 Oct;16(5):493-7. doi: 10.1016/j.copbio.2005.08.002. Curr Opin Biotechnol. 2005. PMID: 16098737 Review.
-
Hydrogel-based non-autologous cell and tissue therapy.Biotechniques. 2000 Sep;29(3):564-72, 574, 576 passim. doi: 10.2144/00293rv01. Biotechniques. 2000. PMID: 10997271 Review.
References
-
- Birch M. P., Messenger A. G. Genetic factors predispose to balding and non-balding in men. European Journal of Dermatology. 2001;11(4):309–314. - PubMed
-
- Horne K. A., Jahoda C. A., Oliver R. F. Whisker growth induced by implantation of cultured vibrissa dermal papilla cells in the adult rat. Journal of Embryology and Experimental Morphology. 1986;97:111–124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

