Rilpivirine and Doravirine Have Complementary Efficacies Against NNRTI-Resistant HIV-1 Mutants
- PMID: 27124362
- PMCID: PMC4942337
- DOI: 10.1097/QAI.0000000000001031
Rilpivirine and Doravirine Have Complementary Efficacies Against NNRTI-Resistant HIV-1 Mutants
Abstract
Background: Rilpivirine (RPV) is the latest non-nucleoside reverse transcriptase inhibitor (NNRTI) to be approved by Food and Drug Administration to combat HIV-1 infections. NNRTIs inhibit the chemical step in viral DNA synthesis by binding to an allosteric site located about 10 Å from the polymerase active site of reverse transcriptase (RT). Although NNRTIs potently inhibit the replication of wild-type HIV-1, the binding site is not conserved, and mutations arise in the binding pocket. Doravirine (DOR) is a new NNRTI in phase III clinical trials.
Methods: Using a single round HIV-1 infection assay, we tested RPV and DOR against a broad panel of NNRTI-resistant mutants to determine their respective activities. We also used molecular modeling to determine if the susceptibility profile of each compound was related to how they bind RT.
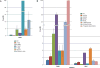
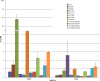
Results: Several mutants displayed decreased susceptibility to DOR. However, with the exception of E138K, our data suggest that the mutations that reduce the potency of DOR and RPV are non-overlapping. Thus, these 2 NNRTIs have the potential to be used together in combination therapy. We also show that the location at which DOR and RPV bind with the NNRTI binding pocket of RT correlates with the differences in their respective susceptibility to the panel of NNRTI-resistance mutations.
Conclusions: This shows that (1) DOR is susceptible to a number of well-known NNRTI resistance mutations and (2) an understanding of the mutational susceptibilities and binding interactions of NNRTIs with RT could be used to develop pairs of compounds with non-overlapping mutational susceptibilities.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

