Hypoxic control of metastasis
- PMID: 27124451
- PMCID: PMC4898055
- DOI: 10.1126/science.aaf4405
Hypoxic control of metastasis
Abstract
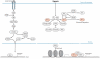
Metastatic disease is the leading cause of cancer-related deaths and involves critical interactions between tumor cells and the microenvironment. Hypoxia is a potent microenvironmental factor promoting metastatic progression. Clinically, hypoxia and the expression of the hypoxia-inducible transcription factors HIF-1 and HIF-2 are associated with increased distant metastasis and poor survival in a variety of tumor types. Moreover, HIF signaling in malignant cells influences multiple steps within the metastatic cascade. Here we review research focused on elucidating the mechanisms by which the hypoxic tumor microenvironment promotes metastatic progression. These studies have identified potential biomarkers and therapeutic targets regulated by hypoxia that could be incorporated into strategies aimed at preventing and treating metastatic disease.
Copyright © 2016, American Association for the Advancement of Science.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

