Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio
- PMID: 27124460
- PMCID: PMC4850741
- DOI: 10.1126/science.aad4017
Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio
Abstract
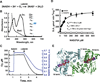
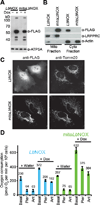
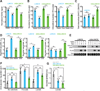
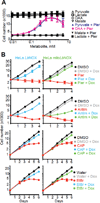
A decline in electron transport chain (ETC) activity is associated with many human diseases. Although diminished mitochondrial adenosine triphosphate production is recognized as a source of pathology, the contribution of the associated reduction in the ratio of the amount of oxidized nicotinamide adenine dinucleotide (NAD(+)) to that of its reduced form (NADH) is less clear. We used a water-forming NADH oxidase from Lactobacillus brevis (LbNOX) as a genetic tool for inducing a compartment-specific increase of the NAD(+)/NADH ratio in human cells. We used LbNOX to demonstrate the dependence of key metabolic fluxes, gluconeogenesis, and signaling on the cytosolic or mitochondrial NAD(+)/NADH ratios. Expression of LbNOX in the cytosol or mitochondria ameliorated proliferative and metabolic defects caused by an impaired ETC. The results underscore the role of reductive stress in mitochondrial pathogenesis and demonstrate the utility of targeted LbNOX for direct, compartment-specific manipulation of redox state.
Copyright © 2016, American Association for the Advancement of Science.
Figures
References
-
- Vafai SB, Mootha VK. Mitochondrial disorders as windows into an ancient organelle. Nature. 2012 Nov 15;491:374. - PubMed
-
- Hummel W, Riebel B. Isolation and biochemical characterization of a new NADH oxidase from Lactobacillus brevis. Biotechnology letters. 2003 Jan;25:51. - PubMed
-
- Lopez de Felipe F, Hugenholtz J. Purification and characterisation of the water forming NADH-oxidase from Lactococcus lactis. International Dairy Journal. 2001;11:37.
-
- Yu J, et al. Characterization of the Streptococcus pneumoniae NADH oxidase that is required for infection. Microbiology. 2001 Feb;147:431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

