Bottom-Up Colloidal Crystal Assembly with a Twist
- PMID: 27124487
- PMCID: PMC4881195
- DOI: 10.1021/acsnano.6b01854
Bottom-Up Colloidal Crystal Assembly with a Twist
Abstract
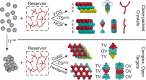
Globally ordered colloidal crystal lattices have broad utility in a wide range of optical and catalytic devices, for example, as photonic band gap materials. However, the self-assembly of stereospecific structures is often confounded by polymorphism. Small free-energy differences often characterize ensembles of different structures, making it difficult to produce a single morphology at will. Current techniques to handle this problem adopt one of two approaches: that of the "top-down" or "bottom-up" methodology, whereby structures are engineered starting from the largest or smallest relevant length scales, respectively. However, recently, a third approach for directing high fidelity assembly of colloidal crystals has been suggested which relies on the introduction of polymer cosolutes into the crystal phase [Mahynski, N.; Panagiotopoulos, A. Z.; Meng, D.; Kumar, S. K. Nat. Commun. 2014, 5, 4472]. By tuning the polymer's morphology to interact uniquely with the void symmetry of a single desired crystal, the entropy loss associated with polymer confinement has been shown to strongly bias the formation of that phase. However, previously, this approach has only been demonstrated in the limiting case of close-packed crystals. Here, we show how this approach may be generalized and extended to complex open crystals, illustrating the utility of this "structure-directing agent" paradigm in engineering the nanoscale structure of ordered colloidal materials. The high degree of transferability of this paradigm's basic principles between relatively simple crystals and more complex ones suggests that this represents a valuable addition to presently known self-assembly techniques.
Keywords: colloidal crystals; colloids; crystal polymorphism; polymers; self-assembly; tetrastack.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Lovallo M. C.; Tsapatsis M. Preferentially Oriented Submicron Silicalite Membranes. AIChE J. 1996, 42, 3020–3029. 10.1002/aic.690421104. - DOI
-
- Yang Y.; Bolikal D.; Becker M. L.; Kohn J.; Zeiger D. N.; Simon C. G. Jr. Combinatorial Polymer Scaffold Libraries for Screening Cell-Biomaterial Interactions in 3D. Adv. Mater. 2008, 20, 2037–2043. 10.1002/adma.200702088. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

