Early CD4+ T Cell Responses Are Associated with Subsequent CD8+ T Cell Responses to an rAd5-Based Prophylactic Prime-Boost HIV Vaccine Strategy
- PMID: 27124598
- PMCID: PMC4849671
- DOI: 10.1371/journal.pone.0152952
Early CD4+ T Cell Responses Are Associated with Subsequent CD8+ T Cell Responses to an rAd5-Based Prophylactic Prime-Boost HIV Vaccine Strategy
Abstract
Introduction: Initial evaluation of a candidate vaccine against HIV includes an assessment of the vaccine's ability to generate immune responses. However, the dynamics of vaccine-induced immune responses are unclear. We hypothesized that the IFN-γ producing cytotoxic CD8+ (CD8+ IFN-γ+) T cell responses could be predicted by early IL-2 producing CD4+ (CD4+ IL-2+) helper T cell responses, and we evaluated this hypothesis using data from a phase I/II prophylactic HIV vaccine trial. The objective was to assess the dynamics and correlations between CD4+ IL-2+ T cell and CD8+ IFN-γ+ T cell responses after vaccination with a recombinant adenoviral serotype 5 (rAd5) HIV vaccine.
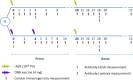
Methods: We analyzed data from the HVTN 068 HIV vaccine trial, which evaluated the immunogenicity of two different strategies for prime and boost vaccination (rAd5-rAd5 vaccine versus DNA-rAd5) in 66 healthy volunteers. Spearman correlations between immunogenicity markers across time-points were calculated. CD8+ IFN-γ+ T cell response in the rAd5-rAd5 arm was modeled as a function of CD4+ IL-2+ T cell response and time using mixed effects regression models.
Results: Moderate to high correlations (r = 0.48-0.76) were observed in the rAd5-rAd5 arm between the CD4+ IL-2+ T cell response at week 2 and later CD8+ IFN-γ+ T cell responses (weeks 2-52). Regression models confirmed this relationship with a significant association between the two markers: for a 1.0% increase in CD4+ IL-2+ T cells at week 2 post-prime, a 0.3% increase in CD8+ IFN-γ+ T cell responses across subsequent time points, including post-boost time points, was observed (p<0.01).
Conclusion: These results suggest an early and leading role of CD4+ T cells in the cellular response to the rAd5-rAd5 vaccine and in particular the stimulation of cytotoxic CD8+ T cell responses. These results could inform better timing of CD4+ T cell measurements in future clinical trials.
Conflict of interest statement
Figures


Similar articles
-
Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses.PLoS One. 2010 Feb 2;5(2):e9015. doi: 10.1371/journal.pone.0009015. PLoS One. 2010. PMID: 20126394 Free PMC article. Clinical Trial.
-
Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa.PLoS One. 2010 Sep 21;5(9):e12873. doi: 10.1371/journal.pone.0012873. PLoS One. 2010. PMID: 20877623 Free PMC article. Clinical Trial.
-
Safety and Immunogenicity of a rAd35-EnvA Prototype HIV-1 Vaccine in Combination with rAd5-EnvA in Healthy Adults (VRC 012).PLoS One. 2016 Nov 15;11(11):e0166393. doi: 10.1371/journal.pone.0166393. eCollection 2016. PLoS One. 2016. PMID: 27846256 Free PMC article. Clinical Trial.
-
Multiple Approaches for Increasing the Immunogenicity of an Epitope-Based Anti-HIV Vaccine.AIDS Res Hum Retroviruses. 2015 Nov;31(11):1077-88. doi: 10.1089/AID.2015.0101. Epub 2015 Aug 13. AIDS Res Hum Retroviruses. 2015. PMID: 26149745 Review.
-
T cell immune responses to HIV-1.Front Biosci. 2007 Jan 1;12:2330-43. doi: 10.2741/2235. Front Biosci. 2007. PMID: 17127243 Review.
Cited by
-
First-in-Human Evaluation of the Safety and Immunogenicity of an Intranasally Administered Replication-Competent Sendai Virus-Vectored HIV Type 1 Gag Vaccine: Induction of Potent T-Cell or Antibody Responses in Prime-Boost Regimens.J Infect Dis. 2017 Jan 1;215(1):95-104. doi: 10.1093/infdis/jiw500. Epub 2016 Oct 17. J Infect Dis. 2017. PMID: 28077588 Free PMC article. Clinical Trial.
References
-
- Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983;220(4599): 868–71. - PubMed
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS report on the global AIDS epidemic. Geneva, Switzerland: 2013.
-
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008;372(9653): 1881–93. 10.1016/S0140-6736(08)61591-3 - DOI - PMC - PubMed
-
- Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 2005;191(5): 654–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

