Maintenance of Efficacy and Continuing Safety of Golimumab for Active Ulcerative Colitis: PURSUIT-SC Maintenance Study Extension Through 1 Year
- PMID: 27124701
- PMCID: PMC4855165
- DOI: 10.1038/ctg.2016.24
Maintenance of Efficacy and Continuing Safety of Golimumab for Active Ulcerative Colitis: PURSUIT-SC Maintenance Study Extension Through 1 Year
Abstract
Objectives: The safety and efficacy of subcutaneous golimumab through 2 years of maintenance therapy was evaluated in patients with moderate-to-severe ulcerative colitis (UC).
Methods: Patients completing treatment through week 52 (placebo, golimumab 50, 100, every-4-weeks (q4w)) and evaluations at week 54 were eligible for this long-term extension (LTE) trial. Patients receiving placebo or golimumab 50 mg with worsening disease during the LTE could receive golimumab 100 mg. Efficacy assessments included the Mayo physician's global assessment (PGA) subscore, inflammatory bowel disease questionnaire (IBDQ), and corticosteroid use. Patients who were randomized to golimumab at PURSUIT-Maintenance baseline and continued receiving golimumab during the LTE were analyzed for efficacy (using intention-to-treat and "as observed" analyses; N=195) and safety (N=200). Patients treated with golimumab at any time from induction baseline through week 104 (N=1240) constituted the overall safety population.
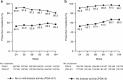
Results: Baseline demographics and disease characteristics of patients entering the LTE receiving golimumab were similar to those of all patients randomized to golimumab maintenance at baseline. At week 104, 80.5% (157/195) of patients had a PGA=0/1 (range weeks 56-104: 80.5-91.8%) and 56.4% (110/195) had a PGA=0 (weeks 56-104: range: 53.8-58.5%). Through week 104, 86% of patients maintained inactive or mild disease activity. Among 174 corticosteroid-free patients at week 54, 88.5% remained corticosteroid-free at week 104. At week 104, 62.2% (120/193) had an IBDQ score ≥170. Tuberculosis, opportunistic infection, and malignancy rates were low, and the overall safety profile was similar to that reported through week 54. Two non-melanoma skin cancers, one metastatic colon cancer, and two deaths (biventricular heart dysfunction, sepsis) occurred between weeks 54 and 104.
Conclusion: Subcutaneous golimumab q4w through 2 years maintained clinical benefit and reduced corticosteroid use among patients who did well in the maintenance study. No new safety signals were observed.
Conflict of interest statement
Figures



References
-
- Sandborn WJ, Feagan BG, Marano C et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 96–109. - PubMed
-
- Sandborn WJ, Feagan BG, Marano C et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 85–95. - PubMed
-
- Reinisch W, Sandborn WJ, Rutgeerts P et al. Long-term infliximab maintenance therapy for ulcerative colitis: the act-1 and -2 extension studies. Inflamm Bowel Dis 2012; 18: 201–211. - PubMed
-
- Irvine EJ, Feagan B, Rochon J et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology 1994; 106: 287–296. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

