Influenza-Specific Antibody-Dependent Phagocytosis
- PMID: 27124730
- PMCID: PMC4849649
- DOI: 10.1371/journal.pone.0154461
Influenza-Specific Antibody-Dependent Phagocytosis
Abstract
Background: Immunity to human influenza A virus (IAV) infection is only partially understood. Broadly non-neutralizing antibodies may assist in reducing disease but have not been well characterized.
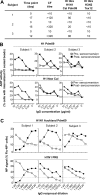
Methods: We measured internalization of opsonized, influenza protein-coated fluorescent beads and live IAV into a monocytic cell line to study antibody-dependent phagocytosis (ADP) against multiple influenza hemagglutinin (HA) subtypes. We analyzed influenza HA-specific ADP in healthy human donors, in preparations of intravenous immunoglobulin (IVIG), and following IAV infection of humans and macaques.
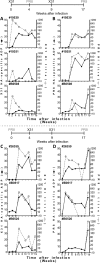
Results: We found that both sera from healthy adults and IVIG preparations had broad ADP to multiple seasonal HA proteins and weak cross-reactive ADP to non-circulating HA proteins. The ADP in experimentally influenza-infected macaque plasma and naturally influenza-infected human sera mediated phagocytosis of both homologous and heterologous IAVs. Further, the IAV phagocytosed in an antibody-mediated manner had reduced infectivity in vitro.
Conclusion: We conclude that IAV infections in humans and macaques leads to the development of influenza-specific ADP that can clear IAV infection in vitro. Repeated exposure of humans to multiple IAV infections likely leads to the development of ADP that is cross-reactive to strains not previously encountered. Further analyses of the protective capacity of broadly reactive influenza-specific ADP is warranted.
Conflict of interest statement
Figures





References
-
- WHO. Influenza (Seasonal) 2015 [updated March 2014]. Available: http://www.who.int/mediacentre/factsheets/fs211/en/. Accessed 2015 February 7.
-
- Kilbourne ED, Smith C, Brett I, Pokorny BA, Johansson B, Cox N. The total influenza vaccine failure of 1947 revisited: major intrasubtypic antigenic change can explain failure of vaccine in a post-World War II epidemic. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(16):10748–52. Epub 2002/07/24. 10.1073/pnas.162366899 ; PubMed Central PMCID: PMCPmc125033. - DOI - PMC - PubMed
-
- Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC medicine. 2013;11:153 Epub 2013/06/27. 10.1186/1741-7015-11-153 ; PubMed Central PMCID: PMCPmc3706345. - DOI - PMC - PubMed
-
- Talbot HK, Zhu Y, Chen Q, Williams JV, Thompson MG, Griffin MR. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in adults, 2011–2012 influenza season. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;56(12):1774–7. Epub 2013/03/02. 10.1093/cid/cit124 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

