Effectiveness of the 10-Valent Pneumococcal Nontypeable Haemophilus influenzae Protein D-Conjugated Vaccine (PHiD-CV) Against Carriage and Acute Otitis Media-A Double-Blind Randomized Clinical Trial in Finland
- PMID: 27125273
- PMCID: PMC5125453
- DOI: 10.1093/jpids/piw010
Effectiveness of the 10-Valent Pneumococcal Nontypeable Haemophilus influenzae Protein D-Conjugated Vaccine (PHiD-CV) Against Carriage and Acute Otitis Media-A Double-Blind Randomized Clinical Trial in Finland
Abstract
After administering the 10-valent pneumococcal polysaccharide nontypeable Haemophilus influenzae protein D-conjugated vaccine (PHiD-CV) to children aged 2-18 months, we observed a reduction in vaccine-type nasopharyngeal carriage, resulting in a reduction of overall pneumococcal nasopharyngeal carriage, which may be important for indirect vaccine effects. We noted a trend toward reduction of acute otitis media.
Background: This trial (ClinicalTrials.gov identifier NCT00839254), nested within a cluster-randomized double-blind invasive pneumococcal disease effectiveness study in Finland (ClinicalTrials.gov identifier NCT00861380), assessed the effectiveness of the 10-valent pneumococcal polysaccharide nontypeable Haemophilus influenzae protein D-conjugated vaccine (PHiD-CV or PCV10) against bacterial nasopharyngeal carriage and acute otitis media (AOM).
Methods: Infants (aged 6 weeks to 6 months) received the PHiD-CV or a control vaccine (hepatitis B) (schedule 3+1 or 2+1). Nasopharyngeal swabs were collected at 4 time points post-vaccination from all of the infants and at pre-vaccination from a subset. Parent-reported physician-diagnosed AOM was assessed from first vaccination until last contact (mean follow-up, 18 months). Vaccine effectiveness (VE) was derived as (1 - relative risk)*100, accounting for cluster design in AOM analysis. Significant VE was assessed descriptively (positive lower limit of the non-adjusted 95% confidence interval [CI]).
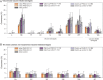
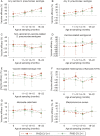
Results: The vaccinated cohort included 5093 infants for carriage assessment and 4117 infants for AOM assessment. Both schedules decreased vaccine-serotype carriage, with a trend toward a lesser effect from the 2+1 schedule ( VE across timpoints 19%-56% [3+1] and 1%-38% [2+1]). Trends toward reduced pneumococcal carriage (predominantly vaccine serotypes 6B, 14, 19F, and 23F), decreased carriage of vaccine-related serotype 19A, and small increases at later time points (ages 14-15 months) in non-vaccine-serotype carriage were observed. No effects on nontypeable Haemophilus influenzae, Staphylococcus aureus, or Moraxella catarrhalis carriage were observed. There were non-significant trends toward a reduction in the number of infants reporting AOM episodes (VE 3+1: 6.1% [95% CI, -2.7% to 14.1%] and 2+1: 7.4% [-2.8% to 16.6%]) and all AOM episodes (VE 3+1: 2.8% [-9.5% to 13.9%] and 2+1: 10.2% [-4.1% to 22.9%]). PHiD-CV was immunogenic and had an acceptable safety profile.
Conclusions: We observed reduced vaccine-type pneumococcal carriage, a limited increase in non-vaccine-type carriage, and a trend toward AOM reduction.
Keywords: PHiD-CV; Streptococcus pneumoniae; acute otitis media; nasopharyngeal carriage; pneumococcal conjugate vaccine.
© The Author 2016. Published by Oxford University Press on behalf of the Pediatric Infectious Diseases Society.
Figures



Comment in
-
Response to Effectiveness of the 10-Valent Pneumococcal Nontypeable Haemophilus influenzae Protein D-Conjugated Vaccine (PHiD-CV) Against Carriage and Acute Otitis Media-A Double-Blind Randomized Clinical Trial in Finland.J Pediatric Infect Dis Soc. 2017 Mar 1;6(1):109-110. doi: 10.1093/jpids/piw074. J Pediatric Infect Dis Soc. 2017. PMID: 28064237 No abstract available.
References
-
- O'Brien KL, Wolfson LJ, Watt JP et al. . Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374:893–902. - PubMed
-
- Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O'Brien KL. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines 2012; 11:841–55. - PubMed
-
- O'Brien KL, Millar EV, Zell ER et al. . Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis 2007; 196:1211–20. - PubMed
-
- Eskola J, Kilpi T, Palmu A et al. . Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001; 344:403–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

