A Validated Risk Score for Venous Thromboembolism Is Predictive of Cancer Progression and Mortality
- PMID: 27125754
- PMCID: PMC4943384
- DOI: 10.1634/theoncologist.2015-0361
A Validated Risk Score for Venous Thromboembolism Is Predictive of Cancer Progression and Mortality
Abstract
Background: Retrospective studies have suggested an association between cancer-associated venous thromboembolism (VTE) and patient survival. We evaluated a previously validated VTE Clinical Risk Score in also predicting early mortality and cancer progression.
Methods: A large, nationwide, prospective cohort study of adults with solid tumors or lymphoma initiating chemotherapy was conducted from 2002 to 2006 at 115 U.S. practice sites. Survival and cancer progression were estimated by the method of Kaplan and Meier. Multivariate analysis was based on Cox regression analysis adjusted for major prognostic factors including VTE itself.
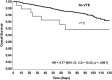
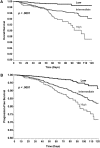
Results: Of 4,405 patients, 134 (3.0%) died and 330 (7.5%) experienced disease progression during the first 4 months of therapy (median follow-up 75 days). Patients deemed high risk (n = 540, 12.3%) by the Clinical Risk Score had a 120-day mortality rate of 12.7% (adjusted hazard ratio [aHR] 3.00, 95% confidence interval [CI] 1.4-6.3), and intermediate-risk patients (n = 2,665, 60.5%) had a mortality rate of 5.9% (aHR 2.3, 95% CI 1.2-4.4) compared with only 1.4% for low-risk patients (n = 1,200, 27.2%). At 120 days of follow-up, cancer progression occurred in 27.2% of high-risk patients (aHR 2.2, 95% CI 1.4-3.5) and 16.4% of intermediate-risk patients (aHR 1.9, 95% CI 1.3-2.7) compared with only 8.5% of low-risk patients (p < .0001).
Conclusion: The Clinical Risk Score, originally developed to predict the occurrence of VTE, is also predictive of early mortality and cancer progression during the first four cycles of outpatient chemotherapy, independent from other major prognostic factors including VTE itself. Ongoing and future studies will help determine the impact of VTE prophylaxis on survival.
Implications for practice: The risk of venous thromboembolism (VTE) is increased in patients receiving cancer chemotherapy. In this article, the authors demonstrate that a popular risk score for VTE in patients with cancer is also associated with the risk of early mortality in this setting. It is important that clinicians evaluate the risk of VTE in patients receiving cancer treatment and discuss the risk and associated symptoms of VTE with patients. Individuals at increased risk should be advised that VTE is a medical emergency and should be urgently diagnosed and appropriately treated to reduce the risk of serious and life-threatening complications.
Keywords: Drug therapy; Mortality; Neoplasms; Outpatients; Survival; Thromboembolism.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Khorana AA, Francis CW, Culakova E, et al. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822–2829. - PubMed
-
- Lyman GH. The incidence of venous thromboembolism in cancer patients: A real-world analysis. Clin Adv Hematol Oncol. 2012;10:40–42. - PubMed
-
- Sørensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. - PubMed
-
- Khorana AA, Rosenblatt JD, Sahasrabudhe DM, et al. A phase I trial of immunotherapy with intratumoral adenovirus-interferon-gamma (TG1041) in patients with malignant melanoma. Cancer Gene Ther. 2003;10:251–259. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

