A monoclonal natural human IgM protects axons in the absence of remyelination
- PMID: 27126523
- PMCID: PMC4850699
- DOI: 10.1186/s12974-016-0561-3
A monoclonal natural human IgM protects axons in the absence of remyelination
Abstract
Background: Whereas demyelination underlies early neurological symptoms in multiple sclerosis (MS), axonal damage is considered critical for permanent chronic deficits. Intracerebral infection of susceptible mouse strains with Theiler's murine encephalomyelitis virus (TMEV) results in chronic induced demyelinating disease (TMEV-IDD) with progressive axonal loss and neurologic dysfunction similar to progressive forms of MS. We previously reported that treatment of chronic TMEV-IDD mice with a neurite outgrowth-promoting natural human antibody, HIgM12, improved brainstem NAA concentrations and preserved functional motor activity. In order to translate this antibody toward clinical trial, we generated a fully human recombinant form of HIgM12, rHIgM12, determined the optimal in vivo dose for functional improvement in TMEV-IDD, and evaluated the functional preservation of descending spinal cord axons by retrograde labeling.
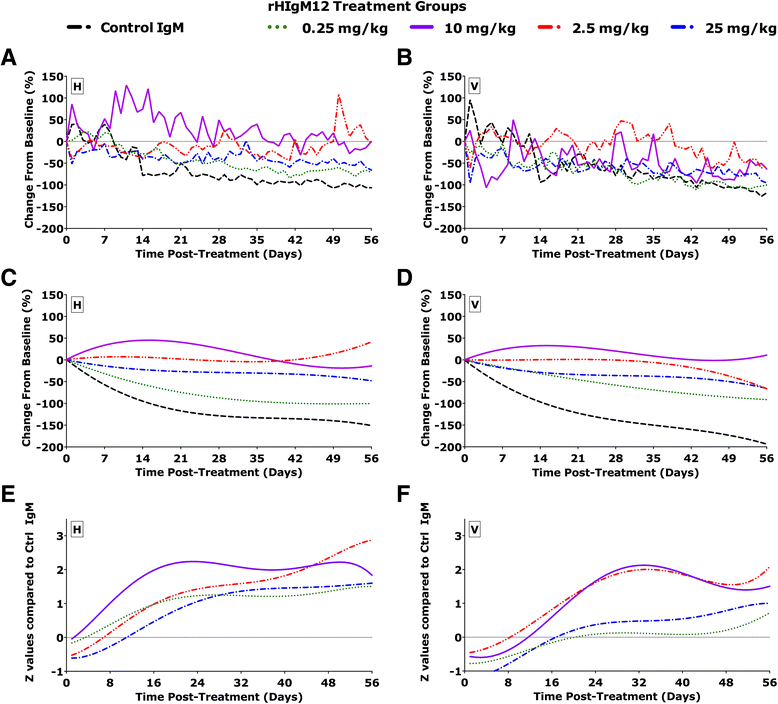
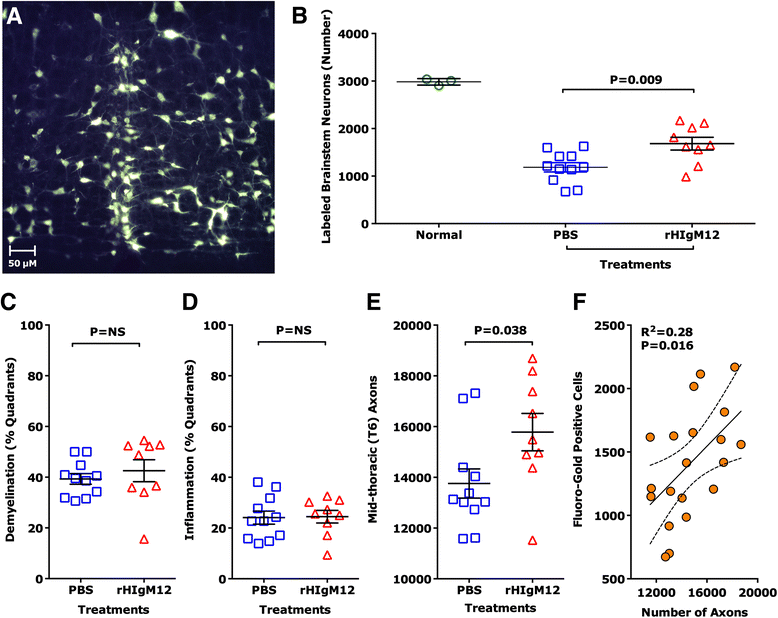
Findings: SJL/J mice at 45 to 90 days post infection (dpi) were studied. A single intraperitoneal dose of 0.25 mg/kg of rHIgM12 per mouse is sufficient to preserve motor function in TMEV-IDD. The optimal dose was 10 mg/kg. rHIgM12 treatment protected the functional transport in spinal cord axons and led to 40 % more Fluoro-Gold-labeled brainstem neurons in retrograde transport studies. This suggests that axons are not only present but also functionally competent. rHIgM12-treated mice also contained more mid-thoracic (T6) spinal cord axons than controls.
Conclusions: This study confirms that a fully human recombinant neurite outgrowth-promoting monoclonal IgM is therapeutic in a model of progressive MS using multiple reparative readouts. The minimum effective dose is similar to that of a remyelination-promoting monoclonal human IgM discovered by our group that is presently in clinical trials for MS.
Keywords: Activity monitoring; Axons; Brainstem; Multiple sclerosis; Retrograde labeling; Theiler’s murine encephalomyelitis virus.
Figures
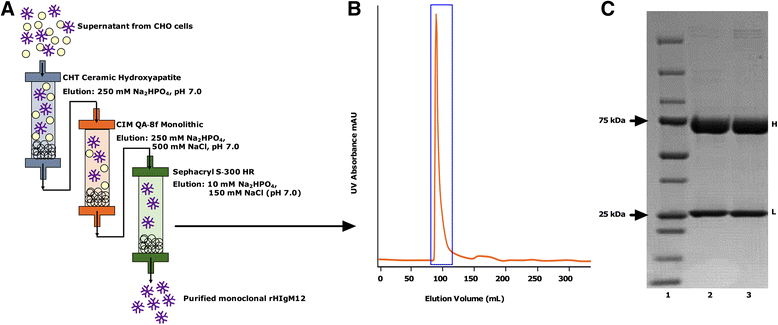
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

