Increased expression of the frontotemporal dementia risk factor TMEM106B causes C9orf72-dependent alterations in lysosomes
- PMID: 27126638
- PMCID: PMC5181637
- DOI: 10.1093/hmg/ddw127
Increased expression of the frontotemporal dementia risk factor TMEM106B causes C9orf72-dependent alterations in lysosomes
Abstract
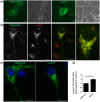
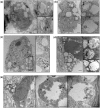
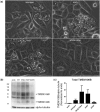
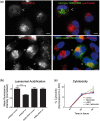
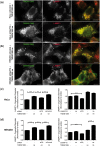
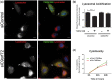
Frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP) is an important cause of dementia in individuals under age 65. Common variants in the TMEM106B gene were previously discovered by genome-wide association to confer genetic risk for FTLD-TDP (p = 1 × 10-11, OR = 1.6). Furthermore, TMEM106B may act as a genetic modifier affecting age at onset and age at death in the Mendelian subgoup of FTLD-TDP due to expansions of the C9orf72 gene. Evidence suggests that TMEM106B variants increase risk for developing FTLD-TDP by increasing expression of Transmembrane Protein 106B (TMEM106B), a lysosomal protein. To further understand the functional role of TMEM106B in disease pathogenesis, we investigated the cell biological effects of increased TMEM106B expression. Here, we report that increased TMEM106B expression results in the appearance of a vacuolar phenotype in multiple cell types, including neurons. Concomitant with the development of this vacuolar phenotype, cells over-expressing TMEM106B exhibit impaired lysosomal acidification and degradative function, as well as increased cytotoxicity. We further identify a potential lysosomal sorting motif for TMEM106B and demonstrate that abrogation of sorting to lysosomes rescues TMEM106B-induced defects. Finally, we show that TMEM106B-induced defects are dependent on the presence of C9orf72, as knockdown of C9orf72 also rescues these defects. In sum, our results suggest that TMEM106B exerts its effects on FTLD-TDP disease risk through alterations in lysosomal pathways. Furthermore, TMEM106B and C9orf72 may interact in FTLD-TDP pathophysiology.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Neary D., Snowden J., Mann D. (2005) Frontotemporal dementia. Lancet Neurol., 4, 771–780. - PubMed
-
- Ratnavalli E., Brayne C., Dawson K., Hodges J.R. (2002) The prevalence of frontotemporal dementia. Neurology, 58, 1615–1621. - PubMed
-
- Baborie A., Griffiths T.D., Jaros E., McKeith I.G., Burn D.J., Richardson A., Ferrari R., Moreno J., Momeni P., Duplessis D, et al. (2011) Pathological correlates of frontotemporal lobar degeneration in the elderly. Acta Neuropathol., 121, 365–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

