3D Bioprinting for Tissue and Organ Fabrication
- PMID: 27126775
- PMCID: PMC5085899
- DOI: 10.1007/s10439-016-1612-8
3D Bioprinting for Tissue and Organ Fabrication
Abstract
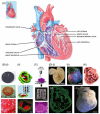
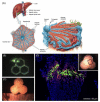
The field of regenerative medicine has progressed tremendously over the past few decades in its ability to fabricate functional tissue substitutes. Conventional approaches based on scaffolding and microengineering are limited in their capacity of producing tissue constructs with precise biomimetic properties. Three-dimensional (3D) bioprinting technology, on the other hand, promises to bridge the divergence between artificially engineered tissue constructs and native tissues. In a sense, 3D bioprinting offers unprecedented versatility to co-deliver cells and biomaterials with precise control over their compositions, spatial distributions, and architectural accuracy, therefore achieving detailed or even personalized recapitulation of the fine shape, structure, and architecture of target tissues and organs. Here we briefly describe recent progresses of 3D bioprinting technology and associated bioinks suitable for the printing process. We then focus on the applications of this technology in fabrication of biomimetic constructs of several representative tissues and organs, including blood vessel, heart, liver, and cartilage. We finally conclude with future challenges in 3D bioprinting as well as potential solutions for further development.
Keywords: Additive manufacturing; Bioink; Bioprinting; Regenerative medicine; Tissue engineering.
Figures



References
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. - PubMed
-
- Langer R, Vacanti JP, Vacanti CA, Atala A, Freed LE, Vunjak-Novakovic G. Tissue engineering: Biomedical applications. Tissue Eng. 1995;1:151–161. - PubMed
-
- Langer R. Tissue engineering: Status and challenges. e-Biomed: J Regen Med. 2000;1:5–6.
-
- Khademhosseini A, Vacanti JP, Langer R. Progress in tissue engineering. Sci Am. 2009;300:64–71. - PubMed
-
- Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Engineering the regenerative microenvironment with biomaterials. Adv Healthc Mater. 2013;2:57–71. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

