Weight Gain and Metabolic Consequences of Risperidone in Young Children With Autism Spectrum Disorder
- PMID: 27126856
- PMCID: PMC4851735
- DOI: 10.1016/j.jaac.2016.02.016
Weight Gain and Metabolic Consequences of Risperidone in Young Children With Autism Spectrum Disorder
Abstract
Objective: We examine weight gain and metabolic consequences of risperidone monotherapy in children with autism spectrum disorder (ASD).
Method: This was a 24-week, multisite, randomized trial of risperidone only versus risperidone plus parent training in 124 children (mean age 6.9 ± 2.35 years; 105 boys and 19 girls) with ASD and serious behavioral problems. We monitored height, weight, waist circumference, and adverse effects during the trial. Fasting blood samples were obtained before treatment and at week 16.
Results: In 97 children with a mean of 22.9 ± 2.8 weeks of risperidone exposure, there was a 5.4 ± 3.4 kg weight gain over 24 weeks (p < .0001); waist circumference increased from 60.7 ± 10.4 cm to 66.8 ± 11.3 cm (p < .0001). At baseline, 59 of 97 children (60.8%) were classified as having normal weight; by week 24, only 25 of 85 (29.4%) remained in that group. Growth curve analysis showed a significant change in body mass index (BMI) z scores from pretreatment to week 24 (p < .0001). This effect was significantly greater for children with reported increased appetite in the first 8 weeks. From before treatment to week 16, there were significant increases in glucose (p = .02), hemoglobin A1c (p = .01), insulin (p <.0001), homeostatic model assessment-insulin resistance (HOMA-IR; p < .001), alanine aminotransferase (p = .01), and leptin (p < .0001). Adiponectin declined (p = .003). At baseline, 7 children met conventional criteria for metabolic syndrome; by week 16, 12 additional children were so classified.
Conclusion: Rapid weight gain with risperidone treatment may promote the cascade of biochemical indices associated with insulin resistance and metabolic syndrome. Appetite, weight, waist circumference, liver function tests, blood lipids, and glucose warrant monitoring. Clinical trial registration information-Drug and Behavioral Therapy for Children With Pervasive Developmental Disorders; http://clinicaltrials.gov/; NCT00080145.
Keywords: autism spectrum disorder; insulin resistance; metabolic syndrome; risperidone; weight gain.
Published by Elsevier Inc.
Figures

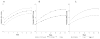
References
-
- Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–321. - PubMed
-
- Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114:e634–41. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text rev.
-
- Research Units on Pediatric Psychopharmacology Autism Network. Risperidone for the core symptom domain of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry. 2005;162:1142–8. - PubMed
-
- Troost PW, Lahuis BE, Steenhuis MP, et al. Long-term effects of risperidone in children with autism spectrum disorders: a placebo discontinuation study. J Am Acad Child Adolesc Psychiatry. 2005;44:1137–44. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

