Prognostic implications of baseline anaemia and changes in haemoglobin concentrations with amphotericin B therapy for cryptococcal meningitis
- PMID: 27126930
- PMCID: PMC5507613
- DOI: 10.1111/hiv.12387
Prognostic implications of baseline anaemia and changes in haemoglobin concentrations with amphotericin B therapy for cryptococcal meningitis
Abstract
Objectives: Anaemia represents a common toxicity with amphotericin B-based induction therapy in HIV-infected persons with cryptococcal meningitis. We sought to examine the impact of amphotericin-related anaemia on survival.
Methods: We used data from Ugandan and South African trial participants to characterize the variation of haemoglobin concentrations from diagnosis to 12 weeks post-diagnosis. Anaemia severity was classified based on the haemoglobin concentration at cryptococcal meningitis diagnosis, and nadir haemoglobin values during amphotericin induction. Cox proportional hazard models were used to estimate 2- and 10-week mortality risk. We also estimated 10-week mortality risk among participants with nadir haemoglobin < 8.5 g/dL during amphotericin induction and who survived ≥ 2 weeks post-enrolment.
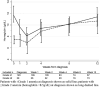
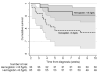
Results: The median haemoglobin concentration at meningitis diagnosis was 11.5 g/dL [interquartile range (IQR) 9.7-13 g/dL; n = 311] with a mean decline of 4.2 g/dL [95% confidence interval (CI) -4.6 to -3.8; P < 0.001; n = 148] from diagnosis to nadir value among participants with baseline haemoglobin ≥ 8.5 g/dL. The median haemoglobin concentration was 8.1 g/dL (IQR 6.5-9.5 g/dL) at 2 weeks, increasing to 9.4 g/dL (IQR 8.2-10.9 g/dL) by 4 weeks and continuing to increase to 12 weeks. Among participants with haemoglobin < 8.5 g/dL at diagnosis, mortality risk was elevated at 2 weeks [hazard ratio (HR) 2.7; 95% CI 1.5-4.9; P < 0.01] and 10 weeks (HR 1.8; 95% CI 1.1-2.2; P = 0.03), relative to those with haemoglobin ≥ 8.5 g/dL. New-onset anaemia occurring with amphotericin therapy did not have a statistically significant association with 10-week mortality (HR 2.0; 95% CI 0.5-9.1; P = 0.4).
Conclusions: Amphotericin induced significant haemoglobin declines, which were mostly transient and did not impact 10-week mortality. Individuals with moderate to life-threatening anaemia at baseline had a higher mortality risk at 2 and 10 weeks post-enrolment.
Keywords: HIV; amphotericin B; anaemia; cryptococcal meningitis.
© 2016 British HIV Association.
Figures

References
-
- Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91:301–308. - PubMed
-
- Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. AIDS. 1999;13:943–950. - PubMed
-
- Berhane K, Karim R, Cohen MH, Masri-Lavine L, Young M, Anastos K, et al. Impact of highly active antiretroviral therapy on anemia and relationship between anemia and survival in a large cohort of HIV-infected women - Women's interagency HIV study. J Acq Imm Def. 2004;37:1245–1252. - PubMed
-
- O'Brien ME, Kupka R, Msamanga GI, Saathoff E, Hunter DJ, Fawzi WW. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acq Imm Def. 2005;40:219–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

