Study of mitochondrial respiratory defects on reprogramming to human induced pluripotent stem cells
- PMID: 27127184
- PMCID: PMC4931846
- DOI: 10.18632/aging.100950
Study of mitochondrial respiratory defects on reprogramming to human induced pluripotent stem cells
Abstract
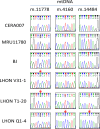
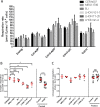
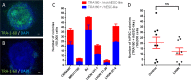
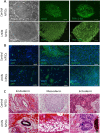
Reprogramming of somatic cells into a pluripotent state is known to be accompanied by extensive restructuring of mitochondria and switch in metabolic requirements. Here we utilized Leber's hereditary optic neuropathy (LHON) as a mitochondrial disease model to study the effects of homoplasmic mtDNA mutations and subsequent oxidative phosphorylation (OXPHOS) defects in reprogramming. We obtained fibroblasts from a total of 6 LHON patients and control subjects, and showed a significant defect in complex I respiration in LHON fibroblasts by high-resolution respiratory analysis. Using episomal vector reprogramming, our results indicated that human induced pluripotent stem cell (hiPSC) generation is feasible in LHON fibroblasts. In particular, LHON-specific OXPHOS defects in fibroblasts only caused a mild reduction and did not significantly affect reprogramming efficiency, suggesting that hiPSC reprogramming can tolerate a certain degree of OXPHOS defects. Our results highlighted the induction of genes involved in mitochondrial biogenesis (TFAM, NRF1), mitochondrial fusion (MFN1, MFN2) and glycine production (GCAT) during reprogramming. However, LHON-associated OXPHOS defects did not alter the kinetics or expression levels of these genes during reprogramming. Together, our study provides new insights into the effects of mtDNA mutation and OXPHOS defects in reprogramming and genes associated with various aspects of mitochondrial biology.
Keywords: Leber's hereditary optic neuropathy; cellular reprogramming; induced pluripotent stem cells; mitochondria; oxidative phosphorylation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Hashizume O, Ohnishi S, Mito T, Shimizu A, Iashikawa K, Nakada K, Soda M, Mano H, Togayachi S, Miyoshi H, Okita K, Hayashi J. Epigenetic regulation of the nuclear-coded GCAT and SHMT2 genes confers human age-associated mitochondrial respiration defects. Scientific reports. 2015;5:10434. - PMC - PubMed
-
- Mertens J, Paquola AC, Ku M, Hatch E, Bohnke L, Ladjevardi S, McGrath S, Campbell B, Lee H, Herdy JR, Goncalves JT, Toda T, Kim Y, et al. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal. Age-Related Nucleocytoplasmic Defects. Cell Stem Cell. 2015;17:705–718. - PMC - PubMed
-
- Quiros PM, Langer T, Lopez-Otin C. New roles for mitochondrial proteases in health, ageing and disease. Nature reviews Molecular cell biology. 2015;16:345–359. - PubMed
-
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

