Sex Differences in Renal Proximal Tubular Cell Homeostasis
- PMID: 27127188
- PMCID: PMC5042665
- DOI: 10.1681/ASN.2015080886
Sex Differences in Renal Proximal Tubular Cell Homeostasis
Abstract
Studies in human patients and animals have revealed sex-specific differences in susceptibility to renal diseases. Because actions of female sex hormones on normal renal tissue might protect against damage, we searched for potential influences of the female hormone cycle on basic renal functions by studying excretion of urinary marker proteins in healthy human probands. We collected second morning spot urine samples of unmedicated naturally ovulating women, postmenopausal women, and men daily and determined urinary excretion of the renal tubular enzymes fructose-1,6-bisphosphatase and glutathione-S-transferase-α Additionally, we quantified urinary excretion of blood plasma proteins α1-microglobulin, albumin, and IgG. Naturally cycling women showed prominent peaks in the temporal pattern of urinary fructose-1,6-bisphosphatase and glutathione-S-transferase-α release exclusively within 7 days after ovulation or onset of menses. In contrast, postmenopausal women and men showed consistently low levels of urinary fructose-1,6-bisphosphatase excretion over comparable periods. We did not detect changes in urinary α1-microglobulin, albumin, or IgG excretion. Results of this study indicate that proximal tubular tissue architecture, representing a nonreproductive organ-derived epithelium, undergoes periodical adaptations phased by the female reproductive hormone cycle. The temporally delimited higher rate of enzymuria in ovulating women might be a sign of recurring increases of tubular cell turnover that potentially provide enhanced repair capacity and thus, higher resistance to renal damage.
Keywords: nonreproductive functions, urinary marker proteins, Fructose-1,6-bisphosphatase, menstrual cycle; renal proximal tubule cell, sex and gender difference, sex hormones.
Copyright © 2016 by the American Society of Nephrology.
Figures
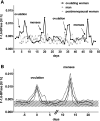

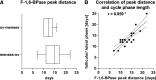




Comment in
-
Sex Differences and Renal Protection: Keeping in Touch with Your Feminine Side.J Am Soc Nephrol. 2016 Oct;27(10):2921-2924. doi: 10.1681/ASN.2016040454. Epub 2016 May 17. J Am Soc Nephrol. 2016. PMID: 27188841 Free PMC article. No abstract available.
References
-
- Committee on Understanding the Biology of Sex and Gender Differences : Board on Health Sciences Policy: Exploring the Biological Contributions to Human Health: Does Sex Matter?, Washington, DC, National Academies Press, 2001 - PubMed
-
- Anonymous: Putting gender on the agenda. Nature 465: 665, 2010 - PubMed
-
- Anonymous: NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research, 2001. Available at: http://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_200.... Accessed May 4, 2015
-
- United States Renal Data System: 2014 USRDS Annual Data Report: An Overview of the Epidemiology of Kidney Disease in the United States, 2014. Available at: http://www.usrds.org. Accessed November 30, 2014
-
- Kramer A, Stel V, Zoccali C, Heaf J, Ansell D, Grönhagen-Riska C, Leivestad T, Simpson K, Pálsson R, Postorino M, Jager K ERA-EDTA Registry : An update on renal replacement therapy in Europe: ERA-EDTA Registry data from 1997 to 2006. Nephrol Dial Transplant 24: 3557–3566, 2009 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

