Magnetic Nanoparticles for Targeting and Imaging of Stem Cells in Myocardial Infarction
- PMID: 27127519
- PMCID: PMC4834159
- DOI: 10.1155/2016/4198790
Magnetic Nanoparticles for Targeting and Imaging of Stem Cells in Myocardial Infarction
Abstract
Stem cell therapy has broad applications in regenerative medicine and increasingly within cardiovascular disease. Stem cells have emerged as a leading therapeutic option for many diseases and have broad applications in regenerative medicine. Injuries to the heart are often permanent due to the limited proliferation and self-healing capability of cardiomyocytes; as such, stem cell therapy has become increasingly important in the treatment of cardiovascular diseases. Despite extensive efforts to optimize cardiac stem cell therapy, challenges remain in the delivery and monitoring of cells injected into the myocardium. Other fields have successively used nanoscience and nanotechnology for a multitude of biomedical applications, including drug delivery, targeted imaging, hyperthermia, and tissue repair. In particular, superparamagnetic iron oxide nanoparticles (SPIONs) have been widely employed for molecular and cellular imaging. In this mini-review, we focus on the application of superparamagnetic iron oxide nanoparticles in targeting and monitoring of stem cells for the treatment of myocardial infarctions.
Figures



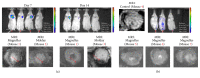

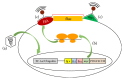
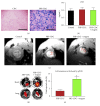
Similar articles
-
The Emergence of Nanotechnology in the Prognosis and Treatment of Myocardial Infarctions.Recent Pat Nanotechnol. 2025;19(1):35-55. doi: 10.2174/1872210517666230721123453. Recent Pat Nanotechnol. 2025. PMID: 37904554 Review.
-
Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: opportunities and challenges.Expert Opin Drug Deliv. 2014 Sep;11(9):1449-70. doi: 10.1517/17425247.2014.924501. Epub 2014 May 29. Expert Opin Drug Deliv. 2014. PMID: 24870351 Review.
-
Targeted extracellular vesicle delivery systems employing superparamagnetic iron oxide nanoparticles.Acta Biomater. 2021 Oct 15;134:13-31. doi: 10.1016/j.actbio.2021.07.027. Epub 2021 Jul 18. Acta Biomater. 2021. PMID: 34284151 Review.
-
Triple Therapy of HER2+ Cancer Using Radiolabeled Multifunctional Iron Oxide Nanoparticles and Alternating Magnetic Field.Cancer Biother Radiopharm. 2016 Nov;31(9):324-329. doi: 10.1089/cbr.2016.2068. Cancer Biother Radiopharm. 2016. PMID: 27831759
-
Superparamagnetic Iron Oxide Nanoparticles in Musculoskeletal Biology.Tissue Eng Part B Rev. 2017 Aug;23(4):373-385. doi: 10.1089/ten.TEB.2016.0437. Epub 2017 Jan 11. Tissue Eng Part B Rev. 2017. PMID: 27998240 Free PMC article.
Cited by
-
Coupling of transient near infrared photonic with magnetic nanoparticle for potential dissipation-free biomedical application in brain.Sci Rep. 2016 Jul 28;6:29792. doi: 10.1038/srep29792. Sci Rep. 2016. PMID: 27465276 Free PMC article.
-
A brief review of cytotoxicity of nanoparticles on mesenchymal stem cells in regenerative medicine.Int J Nanomedicine. 2019 May 24;14:3875-3892. doi: 10.2147/IJN.S205574. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31213807 Free PMC article. Review.
-
Iron Oxide Nanoparticles in Regenerative Medicine and Tissue Engineering.Nanomaterials (Basel). 2021 Sep 8;11(9):2337. doi: 10.3390/nano11092337. Nanomaterials (Basel). 2021. PMID: 34578651 Free PMC article. Review.
-
Recent Developments in Nanomaterials-Based Drug Delivery and Upgrading Treatment of Cardiovascular Diseases.Int J Mol Sci. 2022 Jan 26;23(3):1404. doi: 10.3390/ijms23031404. Int J Mol Sci. 2022. PMID: 35163328 Free PMC article. Review.
-
Magnetic Nanoparticle Coating Decreases the Senescence and Increases the Targeting Potential of Fibroblasts and Adipose-Derived Mesenchymal Stem Cells.ACS Omega. 2023 Jun 22;8(26):23953-23963. doi: 10.1021/acsomega.3c02449. eCollection 2023 Jul 4. ACS Omega. 2023. PMID: 37426224 Free PMC article.
References
-
- Fryar C. D., Chen T.-C., Li X. Prevalence of Uncontrolled Risk Factors for Cardiovascular Disease: United States, 1999–2010. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2012.
-
- Urbanek K., Rota M., Cascapera S., et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circulation Research. 2005;97(7):663–673. doi: 10.1161/01.RES.0000183733.53101.11. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

