Kinetics of Langerhans cell chimerism in the skin of dogs following 2 Gy TBI allogeneic hematopoietic stem cell transplantation
- PMID: 27127633
- PMCID: PMC4848868
- DOI: 10.1186/s12878-016-0050-z
Kinetics of Langerhans cell chimerism in the skin of dogs following 2 Gy TBI allogeneic hematopoietic stem cell transplantation
Abstract
Background: Langerhans cells (LC) are bone marrow-derived cells in the skin. The LC donor/recipient chimerism is assumed to influence the incidence and severity of graft-versus-host disease (GVHD) after hematopoietic stem cell transplantation (HSCT). In nonmyeloablative (NM) HSCT the appearance of acute GVHD is delayed when compared with myeloablative conditioning. Therefore, we examined the development of LC chimerism in a NM canine HSCT model.
Methods: 2 Gy conditioned dogs received bone marrow from dog leukocyte antigen identical littermates. Skin biopsies were obtained pre- and post-transplant. LC isolation was performed by immunomagnetic separation and chimerism analysis by PCR analyzing variable-number-of-tandem-repeat markers with subsequent capillary electrophoresis.
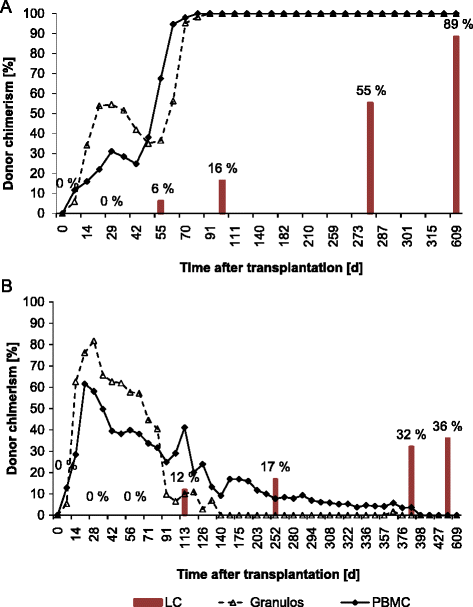
Results: All dogs engrafted. Compared to peripheral blood chimerism the development of LC chimerism was delayed (earliest at day +56). None of the dogs achieved complete donor LC chimerism, although two dogs manifested a 100 % donor chimerism in peripheral blood at days +91 and +77. Of interest, one dog remained LC chimeric despite loss of donor chimerism in the peripheral blood cells.
Conclusion: Our study indicates that LC donor chimerism correlates with chimerism development in the peripheral blood but occurs delayed following NM-HSCT.
Keywords: Chimerism; Dogs; Langerhans cells; Nonmyeloablative; Stem cell transplantation.
Figures

References
-
- Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–63. - PubMed
-
- McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.V97.11.3390. - DOI - PubMed
-
- Khouri IF, Keating M, Körbling M, Przepiorka D, Anderlini P, O’Brien S, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16:2817–24. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

