S100A4 drives non-small cell lung cancer invasion, associates with poor prognosis, and is effectively targeted by the FDA-approved anti-helminthic agent niclosamide
- PMID: 27127879
- PMCID: PMC5085181
- DOI: 10.18632/oncotarget.8969
S100A4 drives non-small cell lung cancer invasion, associates with poor prognosis, and is effectively targeted by the FDA-approved anti-helminthic agent niclosamide
Abstract
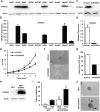
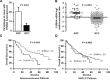
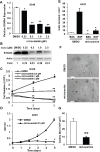
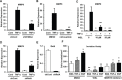
S100A4 (metastasin-1), a metastasis-associated protein and marker of the epithelial to mesenchymal transition, contributes to several hallmarks of cancer and has been implicated in the progression of several types of cancer. However, the impacts of S100A4 signaling in lung cancer progression and its potential use as a target for therapy in lung cancer have not been properly explored. Using established lung cancer cell lines, we demonstrate that S100A4 knockdown reduces cell proliferation, invasion and three-dimensional invasive growth, while overexpression of S100A4 increases invasive potential. In patient-derived tissues, S100A4 is preferentially elevated in lung adenocarcinoma. This elevation is associated with lymphovascular invasion and decreased overall survival. In addition, depletion of S100A4 by shRNA inhibits NF-κB activity and decreases TNFα-induced MMP9 expression. Furthermore, inhibition of the NF-κB/MMP9 axis decreases lung carcinoma invasive potential. Niclosamide, a reported inhibitor of S100A4, blocks expression and function of S100A4 with a reduction in proliferation, invasion and NF-κB-mediated MMP9 expression. Collectively, this study highlights the importance of the S100A4/NF-κB/MMP9 axis in lung cancer invasion and provides a rationale for targeting S100A4 to combat lung cancer.
Keywords: FSP-1; MMP9; NF-κB; NSCLC; metastasin-1.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Robbins SL, Kumar V, Cotran RS. Philadelphia, PA: Saunders/Elsevier; 2010. Robbins and Cotran pathologic basis of disease.
-
- Remon J, Moran T, Majem M, Reguart N, Dalmau E, Marquez-Medina D, Lianes P. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: A new era begins. Cancer treatment reviews. 2014;40:93–101. - PubMed
-
- Lo JF, Yu CC, Chiou SH, Huang CY, Jan CI, Lin SC, Liu CJ, Hu WY, Yu YH. The epithelial-mesenchymal transition mediator S100A4 maintains cancer-initiating cells in head and neck cancers. Cancer research. 2011;71:1912–1923. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

