Disparate response of articular- and auricular-derived chondrocytes to oxygen tension
- PMID: 27128439
- PMCID: PMC4984267
- DOI: 10.1080/03008207.2016.1182996
Disparate response of articular- and auricular-derived chondrocytes to oxygen tension
Abstract
Purpose/aim: To determine the effect of reduced (5%) oxygen tension on chondrogenesis of auricular-derived chondrocytes. Currently, many cell and tissue culture experiments are performed at 20% oxygen with 5% carbon dioxide. Few cells in the body are subjected to this supra-physiological oxygen tension. Chondrocytes and their mesenchymal progenitors are widely reported to have greater chondrogenic expression when cultured at low, more physiological, oxygen tension (1-7%). Although generally accepted, there is still some controversy, and different culture methods, species, and outcome metrics cloud the field. These results are, however, articular chondrocyte biased and have not been reported for auricular-derived chondrocytes.
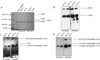
Materials and methods: Auricular and articular chondrocytes were isolated from skeletally mature New Zealand White rabbits, expanded in culture and differentiated in high density cultures with serum-free chondrogenic media. Cartilage tissue derived from aggregate cultures or from the tissue engineered sheets were assessed for biomechanical, glycosaminoglycan, collagen, collagen cross-links, and lysyl oxidase activity and expression.
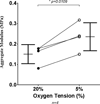
Results: Our studies show increased proliferation rates for both auricular and articular chondrocytes at low (5%) O2 versus standard (20%) O2. In our scaffold-free chondrogenic cultures, low O2 was found to increase articular chondrocyte accumulation of glycosaminoglycan, but not cross-linked type II collagen, or total collagen. Conversely, auricular chondrocytes accumulated less glycosaminoglycan, cross-linked type II collagen and total collagen under low oxygen tension.
Conclusions: This study highlights the dramatic difference in response to low O2 of chondrocytes isolated from different anatomical sites. Low O2 is beneficial for articular-derived chondrogenesis but detrimental for auricular-derived chondrogenesis.
Keywords: Articular chondrocytes; auricular chondrocytes; cartilage tissue engineering; chondrogenesis; collagen cross-linking; oxygen tension.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures








Similar articles
-
Synoviocyte Derived-Extracellular Matrix Enhances Human Articular Chondrocyte Proliferation and Maintains Re-Differentiation Capacity at Both Low and Atmospheric Oxygen Tensions.PLoS One. 2015 Jun 15;10(6):e0129961. doi: 10.1371/journal.pone.0129961. eCollection 2015. PLoS One. 2015. PMID: 26075742 Free PMC article.
-
Characterization of auricular chondrocytes and auricular/articular chondrocyte co-cultures in terms of an application in articular cartilage repair.Int J Mol Med. 2010 May;25(5):701-8. doi: 10.3892/ijmm_00000394. Int J Mol Med. 2010. PMID: 20372812
-
Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes.Cell Tissue Res. 2012 Mar;347(3):649-63. doi: 10.1007/s00441-011-1193-7. Epub 2011 Jun 4. Cell Tissue Res. 2012. PMID: 21638206
-
Glycosaminoglycan-Mediated Interactions in Articular, Auricular, Meniscal, and Nasal Cartilage.Tissue Eng Part B Rev. 2025 Feb;31(1):61-75. doi: 10.1089/ten.TEB.2023.0346. Epub 2024 Apr 22. Tissue Eng Part B Rev. 2025. PMID: 38613808 Review.
-
The chondrocyte.Int J Biochem Cell Biol. 2003 Apr;35(4):401-4. doi: 10.1016/s1357-2725(02)00301-1. Int J Biochem Cell Biol. 2003. PMID: 12565700 Review.
Cited by
-
Optimizing Bioink Composition for Human Chondrocyte Expression of Lubricin.Bioengineering (Basel). 2023 Aug 23;10(9):997. doi: 10.3390/bioengineering10090997. Bioengineering (Basel). 2023. PMID: 37760099 Free PMC article.
-
Physioxia Stimulates Extracellular Matrix Deposition and Increases Mechanical Properties of Human Chondrocyte-Derived Tissue-Engineered Cartilage.Front Bioeng Biotechnol. 2020 Nov 13;8:590743. doi: 10.3389/fbioe.2020.590743. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33282851 Free PMC article.
-
Synoviocyte-Derived Extracellular Matrix and bFGF Speed Human Chondrocyte Proliferation While Maintaining Differentiation Potential.Front Bioeng Biotechnol. 2022 May 24;10:825005. doi: 10.3389/fbioe.2022.825005. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35685088 Free PMC article.
-
High-Throughput, Temporal and Dose Dependent, Effect of Vitamins and Minerals on Chondrogenesis.Front Cell Dev Biol. 2020 Feb 25;8:92. doi: 10.3389/fcell.2020.00092. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32161755 Free PMC article.
-
The Application of Bioreactors for Cartilage Tissue Engineering: Advances, Limitations, and Future Perspectives.Stem Cells Int. 2021 Jan 21;2021:6621806. doi: 10.1155/2021/6621806. eCollection 2021. Stem Cells Int. 2021. PMID: 33542736 Free PMC article. Review.
References
-
- Atala A. Regenerative bladder augmentation using autologous tissue-when will we get there? J Urol. 2014;191:1204–1205. - PubMed
-
- Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117–1124. - PubMed
-
- Gikas PD, Bayliss L, Bentley G, Briggs TW. An overview of autologous chondrocyte implantation. J Bone Joint Surg Br. 2009;91:997–1006. - PubMed
-
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
