Granulocyte transfusions for treating infections in people with neutropenia or neutrophil dysfunction
- PMID: 27128488
- PMCID: PMC4930145
- DOI: 10.1002/14651858.CD005339.pub2
Granulocyte transfusions for treating infections in people with neutropenia or neutrophil dysfunction
Abstract
Background: Despite modern antimicrobials and supportive therapy bacterial and fungal infections are still major complications in people with prolonged disease-related or treatment-related neutropenia. Transfusions of granulocytes have a long history of usage in clinical practice to support and treat severe infection in high-risk groups of patients with neutropenia or neutrophil dysfunction. However, there is considerable current variability in therapeutic granulocyte transfusion practice, and uncertainty about the beneficial effect of transfusions given as an adjunct to antibiotics on mortality. This is an update of a Cochrane review first published in 2005.
Objectives: To determine the effectiveness and safety of granulocyte transfusions compared to no granulocyte transfusions as adjuncts to antimicrobials for treating infections in people with neutropenia or disorders of neutrophil function aimed at reducing mortality and other adverse outcomes related to infection.
Search methods: We searched for randomised controlled trials (RCTs) in the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2016, Issue 2). MEDLINE (from 1946), Embase (from 1974), CINAHL (from 1937), the Transfusion Evidence Library (from 1980) and ongoing trial databases to 11 February 2016.
Selection criteria: RCTs comparing people with neutropenia or disorders of neutrophil dysfunction receiving granulocyte transfusions to treat infection with a control group receiving no granulocyte transfusions. Neonates are the subject of another Cochrane review and were excluded from this review. There was no restriction by outcomes examined, language or publication status.
Data collection and analysis: We used standard methodological procedures expected by the Cochrane Collaboration.
Main results: We identified 10 trials that met the inclusion criteria with a total of 587 participants. We also identified another ongoing trial. These trials were conducted between 1975 and 2015. None of the studies included people with neutrophil dysfunction. The studies differed in the type of infections they included. Six studies included both children and adults, however data were not reported separately for children and adults. The two newest studies gave granulocyte colony stimulating factor (G-CSF) to donors; both were stopped early due to lack of recruitment. Three studies re-randomised participants and therefore quantitative analysis was unable to be performed.Overall the quality of the evidence was very low to low across different outcomes according to GRADE methodology. This was due to many of the studies being at high risk of bias, and many of the outcomes being imprecise.There may be no difference in all-cause mortality over 30 days between participants receiving therapeutic granulocyte transfusions and those that did not (six studies; 321 participants; RR 0.75, 95% CI 0.54 to 1.04; very low-quality evidence). There were no differences between the granulocyte dose subgroups (< 1 x 10(10) per day versus ≥ 1 x 10(10) per day) (test for subgroup differences P = 0.39). There was a difference in all-cause mortality between the studies based on the age of the study (published before 2000 versus published 2000 or later) (test for subgroup differences P = 0.03). There was no difference in all-cause mortality between participants receiving granulocyte transfusions and those that did not in the newest study (one study; 111 participants; RR 1.10, 95% CI 0.70 to 1.73, low-quality evidence). There may be a reduction in all-cause mortality in participants receiving granulocyte transfusions compared to those that did not in studies published before the year 2000 (five studies; 210 participants; RR 0.53, 95% CI 0.33 to 0.85; low-quality evidence).There may be no difference in clinical reversal of concurrent infection between participants receiving therapeutic granulocyte transfusions and those that did not (five studies; 286 participants; RR 0.98, 95% CI 0.81 to 1.19; low-quality evidence).There is insufficient evidence to determine whether there is a difference in pulmonary serious adverse events (1 study; 24 participants; RR 0.85, 95% CI 0.38 to 1.88; very low-quality evidence).None of the studies reported number of days on therapeutic antibiotics, number of adverse events requiring discontinuation of treatment, or quality of life.Six studies reported their funding sources and all were funded by governments or charities.
Authors' conclusions: In people who are neutropenic due to myelosuppressive chemotherapy or a haematopoietic stem cell transplant, there is insufficient evidence to determine whether granulocyte transfusions affect all-cause mortality. To be able to detect a decrease in all-cause mortality from 35% to 30% would require a study containing at least 2748 participants (80% power, 5% significance). There is low-grade evidence that therapeutic granulocyte transfusions may not increase the number of participants with clinical resolution of an infection.
Conflict of interest statement
Lise Estcourt is partly funded by an NIHR Cochrane Programme Grant. Simon Stanworth is involved in the design of clinical trials of granulocytes for transfusion. Carolyn Doree: none known. Sally Hopewell is partly funded by an NIHR Cochrane Programme Grant. Marialena Trivella is partly funded by an NIHR Cochrane Programme Grant. Edwin Massey is involved in the design of clinical trials of granulocytes for transfusion.
Figures
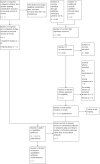
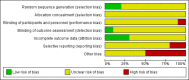


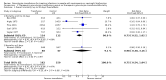






Update of
-
Granulocyte transfusions for treating infections in patients with neutropenia or neutrophil dysfunction.Cochrane Database Syst Rev. 2005 Jul 20;(3):CD005339. doi: 10.1002/14651858.CD005339. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2016 Apr 29;4:CD005339. doi: 10.1002/14651858.CD005339.pub2. PMID: 16034970 Updated.
References
References to studies included in this review
Alavi 1977 {published data only}
-
- Alavi JB, Roor RK, Djerassi I, Evans AE, Gluckman SJ, MacGregor RR, et al. A randomised clinical trial of granulocyte transfusions for infection in acute leukemia. New England Journal of Medicine 1977;296(13):706‐11. - PubMed
Bow 1984 {published data only}
Herzig 1977 {published data only}
-
- Herzig R, Herzig GP, Graw RG, Bull MJ, Ray KK. Successful granulocyte transfusion therapy for gram‐negative septicemia. New England Journal of Medicine 1977;296(13):701‐5. - PubMed
Higby 1975 {published data only}
-
- Higby DJ, Yates JW, Henderson ES, Holland JF. Filtration leukapheresis for granulocyte transfusion therapy. New England Journal of Medicine 1975;292(15):762‐6. - PubMed
Klastersky 1983 {published data only}
-
- EORTC International Antimicrobial Therapy Project Group. Early granulocyte transfusions in high risk febrile neutropenic patients. Schweizerische Medizinische Wochenschrift 1983;113(Suppl. 14):46‐8. - PubMed
-
- EORTC International Antimicrobial Therapy Project Group. Protocol for a cooperative trial of empirical antibiotic treatment and early granulocyte transfusions in febrile neutropenic patients. European Journal of Cancer 1977;13:617‐21. - PubMed
Price 2015 {published data only}
-
- NCT00627393. Safety and effectiveness of granulocyte transfusions in resolving infection in people With neutropenia (The RING Study). www.Clinical Trials.gov 2008 [Accessed 17th February 2015].
-
- Price TH, McCullough J, Ness P, Strauss RG, Pulkrabek SM, Harrison R, et al. A randomized controlled trial on the efficacy of high‐dose granulocyte transfusion therapy in neutropenic patients with infection. Blood 2014;124(21):SCI‐16.
Scali 1978 {published data only}
-
- Scali G, Felten A, Fehr J, Sasuter Chr, Gmòr J. Granulocyte substitution in febrile leukemia patients with bone marrow aplasia. 1. Results of a prospective study [Granulozytensubstitution bei febrilen leukämiepatienten in knochenmarkaplasie]. Schweizerische Medizinische Wochenschrift 1978;108(41):1583‐5. - PubMed
Seidel 2008a {published data only}
-
- Peters C, Seidel MG, Northoff H, Moog R, Boehme A, Silling G, et al. Granulocyte transfusions for treatment or prophylaxis of severe infections in immunocompromized neutropenic patients ‐ a randomized clinical trial. ASH Annual Meeting Abstracts 2006;108(11):2934.
-
- Seidel MG, Peters C, Wacker A, Northoff H, Moog R, Boehme A, et al. Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplantation 2008;42(10):679‐84. - PubMed
Vogler 1977 {published data only}
-
- Vogler WR, Winton EF. A controlled study of the efficacy of granulocyte transfusions in patients with neutropenia. American Journal of Medicine 1977;63:548‐55. - PubMed
Winston 1982a {published data only}
-
- Winston DJ, Winston G, Gale RP. Therapeutic granulocyte transfusions for documented infections ‐ A controlled trial in ninety‐five infectious granulocytopenic episodes. Annals of Internal Medicine 1982;97:509‐15. - PubMed
References to studies excluded from this review
Adkins 1999 {published data only}
-
- Adkins D, Goodnough LT, Moellering J. Reduction in antibiotic utilization and in febrile days by transfusion of G‐CSF mobilised prophylactic granulocyte components: a randomised study. Blood 1999;94:590a.
Altrichter 2011 {published data only}
Ambinder 1981 {published data only}
-
- Ambinder EP, Button GR, Cheung T, Goldberg JD, Holland JF. Filtration versus gravity leukapheresis in febrile granulocytopenic patients: a randomized prospective trial. Blood 1981;57:836‐41. - PubMed
Atay 2011 {published data only}
-
- Atay D, Ozturk G, Akcay A, Yanasik M, Anak S, Devecioglu O. Effect and safety of granulocyte transfusions in pediatric patients with febrile neutropenia or defective granulocyte functions. Journal of Pediatric Hematology/Oncology : Official Journal of the American Society of Pediatric Hematology/Oncology 2011;33(6):e220‐5. - PubMed
Bhatia 1994 {published data only}
-
- Bhatia S, McCullough J, Perry EH, Clay M, Ramsay MKC, Neglia JP. Granulocyte transfusions: efficacy in treating fungal infections in neutropenic patients following bone marrow transplantation. Transfusion 1994;34(3):226‐32. - PubMed
Blum 2001 {published data only}
-
- Blum W, Hallem C, Vij R. Improved survival in allogeneic peripheral blood stem cell transplant patients who received prophylactic granulocyte transfusions from HLA‐matched donors: long term follow up. Blood 2001;98:58a.
Clift 1978 {published data only}
-
- Clift RA, Sanders JE, Thomas ED, Williams B, Buckner CD. Granulocyte transfusions for the prevention of infection in patients receiving bone marrow transplants. New England Journal of Medicine 1978;298:1052‐7. - PubMed
Curtis 1977 {published data only}
Curtis 1982 {published data only}
-
- Curtis J E. Efficacy of granulocyte transfusions collected from non‐stimulated donors. Progress in Clinical and Biological Research 1982;88:61‐73. - PubMed
Diaz 2014 {published data only}
-
- Diaz, R, Soundar E, Hartman SK, Dreyer Z, Teruya J, Hui SK. Granulocyte transfusions for children with infection and neutropenia or granulocyte dysfunction. Pediatric Hematology & Oncology 2014;31(5):425‐34. - PubMed
Ford 1982 {published data only}
-
- Ford JM, Cullen MH, Roberts MM, Brown LM, Oliver RTD, Lister TA. Prophylactic granulocyte transfusions: Results of a randomised controlled trial in patients with acute myelogenous leukemia. Transfusion 1982;22:311‐6. - PubMed
Fortuny 1975 {published data only}
-
- Fortuny IE, Bloomfield CD, Hadlock DC, Goldman A, Kennedy BJ, McCullough JJ. Granulocyte transfusion: a controlled study in patients with acute nonlymphocytic leukemia. Transfusion 1975;15(6):548‐57. - PubMed
Freireich 2013 {published data only}
-
- NCT00968838. A study for leukemia patients with life‐threatening infections. www.clinicaltrials.gov 2008 [Accessed 17th February 2015].
Gomez‐Villagran 1984 {published data only}
-
- Gomez‐Villagran JL, Torres‐Gomez A, Gomez‐Garcia P, Martinez‐Guibelalde F, Velasco‐Jimena F. A controlled trial of prophylactic granulocyte transfusions during induction chemotherapy for acute nonlymphoblastic leukemia. Cancer 1984;54:734‐8. - PubMed
Granena 1978 {published data only}
-
- Grañena A, Aranalde IM, Feliu E, Muncunill J, Montserrat E, Nieto H, Rozman C. The effectiveness of granulocyte transfusions in the treatment of sever injections in granulocytopenic patients. A controlled trial (author's transl) [Eficacia de las transfusiones de granulocitos en el tratemiento de infecciones graves de pacientes granulocitopénicos]. Sangre 1978;23(3):291‐7. - PubMed
Graw 1972 {published data only}
-
- Graw RG, Herzig G, Perry S, Henderson ES. Normal granulocyte transfusion therapy ‐ treatment of septicemia due to gram‐negative bacteria. New England Journal of Medicine 1972;287(8):367‐71. - PubMed
Graw 1977 {published data only}
-
- Graw RG, Stout FG, Herzig RH, Herzig GP. Granulocyte transfusion therapy for life‐threatening bacteremia: results of transfusion trials at the National Cancer Institute. Progress in Clinical and Biological Research 1977;13:267‐80. - PubMed
Hershko 1978 {published data only}
-
- Hershko C, Naparstek E, Eldor A, Izak G. Granulocyte transfusion therapy ‐ a clinical trial in patients with acute leukemia and sepsis. Vox Sanguinis 1978;34:129‐35. - PubMed
Ikemoto 2012 {published data only}
-
- Ikemoto J, Yoshihara S, Fujioka T, Ohtsuka Y, Fujita N, Kokubunji A, et al. Impact of the mobilization regimen and the harvesting technique on the granulocyte yield in healthy donors for granulocyte transfusion therapy. Transfusion 2012;52(12):2646‐52. - PubMed
Illerhaus 2002 {published data only}
-
- Illerhaus G, Wirth K, Dwenger A, Waller CF, Garbe A, Brass V, et al. Treatment and prophylaxis of severe infections in neutropenic patients by granulocyte transfusions. Annals of Hematology 2002;81:273‐81. - PubMed
Mannoni 1979 {published data only}
-
- Mannoni P, Rodet M, Vernant JP, Brun B, Coquin‐Radeau EI, Bracq C, et al. Efficiency of prophylactic granulocyte transfusions in preventing infections in acute leukemia. Revue Francaise de Transfusion et Immuno‐Hematologie 1979;22(5):503‐18. - PubMed
Matsue 1984 {published data only}
-
- Matsue K, Harada M, Nakao S, Ueda M, Kondon K, Odaka K, et al. Controlled study of therapeutic granulocyte transfusion in granulocytopenic patients with severe infections. Japanese Journal of Clinical Oncology 1984;14(1):21‐30. - PubMed
NCT01204788 {published data only}
-
- NCT01204788. Prophylactic white cell transfusions versus therapeutic white cell transfusions in patients with leukemia. www.Clinicaltrials.gov 2010 [Accessed 17 February 2015].
NCT01932710 {published data only}
-
- NCT01932710. A feasibility study of prophylactic white blood cell transfusions. Clinical.Trials.gov 2013:[Accessed 21st April 2015].
Oymak 2015 {published data only}
-
- Oymak Y, Ayhan Y, Karapinar TH, Devrim I, Ay Y, Sarihan H, et al. Granulocyte transfusion experience in pediatric neutropenic fever: Splitted product can be an alternative?. Transfusion & Apheresis Science 2015;53(3):348‐52. - PubMed
Oza 2006 {published data only}
-
- Oza A, Hallemeier C, Goodnough L, Khoury H, Shenoy S, Devine S, et al. Granulocyte‐colony‐stimulating factor‐mobilized prophylactic granulocyte transfusions given after allogeneic peripheral blood progenitor cell transplantation result in a modest reduction of febrile days and intravenous antibiotic usage. Transfusion 2006;46(1):14‐23. [MEDLINE: ] - PubMed
Pammi 2011 {published data only}
Schiffer 1979 {published data only}
-
- Schiffer CA, Aisner J, Daly PA, Schimpff SC, Wiernick PH. Alloimmunisation following prophylactic granulocyte transfusion. Blood 1979;54:766‐74. - PubMed
Stout 2015 {published data only}
-
- Stout IP, Reeves HM, Maitta RW. Analysis of the effectiveness of granulocyte transfusions in neutropenic patients at a large tertiary academic medical center. Transfusion 2015;55:164A.
Strauss 1981 {published data only}
-
- NCT00000581. Granulocyte Transfusion Study. Clinical.Trials.gov 1999:[Accessed 21st April 2015].
-
- Strauss RG, Connett JE, Gale RP, Bloomfield CD, Herzig GP, McCullough J, et al. A controlled trial of prophylactic granulocyte transfusions during initial induction chemotherapy for acute myelogenous leukemia. New England Journal of Medicine 1981;305:597‐603. - PubMed
Strauss 2015 {published data only}
-
- Strauss RG. Neutrophil/granulocyte transfusions collected from G‐CSF + dexamethasone‐stimulated donors. Current Opinion in Hematology 2015;22(6):565‐7. - PubMed
Sutton 1982 {published data only}
-
- Sutton DMC, Shumak KH, Baker MA. Prophylactic granulocyte transfusions in acute leukemia. Plasma Therapy and Transfusion Technology 1982;3:45‐50.
UMIN000014777 {published data only}
-
- UMIN000014777. Study on safety and efficacy of granulocyte transfusion mobilized with G‐CSF in related donor. UMIN Clinical Trials Registry 2014:[Accessed 24th April 2015].
Winston 1982b {published data only}
-
- Winston DJ, Ho WG, Young LS, Gale RP. Prophylactic granulocyte transfusions during human bone marrow transplantation. American Journal of Medicine 1982;68:893‐7. - PubMed
Witt 2015 {published data only}
-
- Witt V, Pichler H. Survey of patients receiving Leukocyte transfusions from 2012 until 2014, a one center retrospective analysis. Transfusion Medicine and Hemotherapy 2015;48(42):9.
Yoshihara 2016 {published data only}
-
- Yoshihara S, Ikemoto J, Fujimori Y. Update on granulocyte transfusions: accumulation of promising data, but still lack of decisive evidence. Current Opinion in Hematology 2016;23(1):55‐60. - PubMed
References to ongoing studies
DRKS00000218 {published data only}
-
- Transfusion of granulocytes for patients with febrile neutropenia. German Clinical Trials Register (DRKS) 2011 (Date Accessed 21 April 2015).
Additional references
Bashir 2003
-
- Bashir S, Cardigan R. Granulocyte concentrates: how can we assess their quality?. Transfusion Medicine 2003;13:245. - PubMed
Bashir 2008
-
- Bashir S, Stanworth S, Massey E, Goddard F, Cardigan R. Neutrophil function is preserved in a pooled granulocyte component prepared from whole blood donations. British Journal of Haematology 2008;140:701‐11. - PubMed
Bennett 2006
-
- Bennett CL, Evens AM, Andritsos LA. Haematological malignancies developing in previously healthy individuals who received haematopoietic growth factors: report from the Research on Adverse Drug Events and Reports (RADAR) project. British Journal of Haematology 2006;135(5):642‐50. - PubMed
Brecher 1953
-
- Brecher G, Wilbur KM, Cronkite EP. Transfusions of separated leukocytes into irradiated dogs with aplastic marrows. Proceedings of the Society for Experimental Biology and Medicine 1953;84:54‐6. - PubMed
Bux 2003
-
- Bux J, Cassens U, Dielschnider T, Duchscherer M, Edel E, Eichler H, et al. Tolerance of granulocyte donors toward granulocyte colony‐stimulating factor stimulation and of patients towards granulocyte transfusions: results of a multicentre study. Vox Sanguinis 2003;85:322‐5. - PubMed
Dale 1976
-
- Dale DC, Reynolds HY, Pennington JE, Elin RJ, Herzig GP. Experimental Pseudomonas pneumonia in leukopenic dogs: comparison of therapy with antibiotics and granulocyte transfusions. Blood 1976;47:869‐76. - PubMed
Dale 2000
-
- Dale DC, Liles WC. Return of granulocyte transfusions. Current Opinion in Pediatrics 2000;12:18‐22. - PubMed
de la Rubia 2008
-
- Rubia J, Arriba F, Arbona C, Pascual MJ, Zamora C, Insunza A, et al. Follow‐up of healthy donors receiving granulocyte colony‐stimulating factor for peripheral blood progenitor cell mobilization and collection. Results of the Spanish Donor Registry. Haematologica 2008;93(5):735‐40. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dellinger 2013
Engelfriet 2000
-
- Engelfriet CO, Reesink HW. Granulocyte transfusions. Vox Sanguinis 2000;79:59‐96. - PubMed
Estcourt 2015
Freireich 1964
-
- Freireich EJ, Levin RH, Whang J, Carbone PP, Bronson W, Morse EE. The function and fate of transfused leukocytes from donors with chronic myelocytic leukemia in leukopenic recipients. Annals of the New York Academy of Sciences 1964;113:1081‐90. - PubMed
Ghodsi 2001
-
- Ghodsi Z, Strauss RG. Cataracts in neutrophil donors stimulated with adrenal corticosteroids. Transfusion 2001;41:1464‐8. - PubMed
Goldman 2006
-
- Goldman JM, Madrigal JA, Pamphilon D. Possible harmful effects of short course granulocyte colony‐stimulating factor in normal donors. British Journal of Haematology 2006 December;8:155‐60. - PubMed
Gutierrez 2001
-
- Gutierrez‐Delgado F, Bensigner W. Safety of granulocyte colony‐stimulating factor in normal donors. Current Opinion in Haematology 2001;8:155‐60. - PubMed
Higgins 2011a
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hubel 2001
-
- Hubel K, Dale DC, Engert A, Liles WC. Current status of granulocyte (neutrophil) transfusion therapy for infectious diseases. Journal of Infectious Diseases 2001;183:321‐8. - PubMed
Hubel 2002
-
- Hubel K, Carter R, Liles WC, dale DC, Price TH, Bowden RA, et al. Granulocyte transfusion therapy for infections in candidates and recipients of hematopoietic stem cell transplant: a comparative analysis of feasibility and outcome of community donors versus related donors. Transfusion 2002;42:1414‐21. - PubMed
Hölig 2013
Kadri 2015
Klastersky 2001
-
- Klastersky J. Empirical treatment of sepsis in neutropenic patients. Hospital Medicine (London) 2001;62(2):101‐3. - PubMed
Kuijpers 1999
-
- Kuijpers TW, Weening RS, Roos D. Clinical and laboratory work‐up of patients with neutrophil shortage or dysfunction. Journal of Immunological Methods. 1999;232:211‐29. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Legrand 2012
-
- Legrand M, Max A, Peigne V, Mariotte E, Canet E, Debrumetz A, et al. Survival in neutropenic patients with severe sepsis or septic shock. Critical Care Medicine 2012;40(1):43‐9. - PubMed
Massey 2012
-
- Massey E, Harding K, Kahan BC, Llewelyn C, Wynn R, Moppett J, et al. The granulocytes in neutropenia 1 (GIN 1) study: a safety study of granulocytes collected from whole blood and stored in additive solution and plasma. Transfusion Medicine 2012;22(4):277‐84. - PubMed
NICE 2012
-
- National Institute for Health and Clinical Excellence (NICE). Neutropenic sepsis: prevention and management of neutropenic sepsis in cancer patients. NICE Guidelines 2012; Vol. CG151. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Review Manager 5.3 [Computer program]
-
- The Nordic Cochrane Centre. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Cochrane Collaboration, 2014.
Robinson 2004
-
- Robinson SP, Marks DI. Granulocyte transfusions in the G‐CSF era. Where do we stand?. Bone Marrow Transplantation 2004;34(10):839‐46. - PubMed
Sacco 2010
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sterne 2011
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stovold 2014
Strauss 1995
-
- Strauss RG. Clinical perspectives of granulocyte transfusions: efficacy to date. Journal of Clinical Apheresis 1995;10:114‐8. - PubMed
Strauss 2003
-
- Strauss RG. Granulocyte (neutrophil) transfusion. Apheresis: Principles and Practice. Bethesda: AABB Press, 2003:237‐52.
Strumia 1934
-
- Strumia MM. The effect of leukocytic cream injections in the treatment of the neutropenias. American Journal of Medicine 1934;187:527‐44.
Tierney 2007
Vamvakas 1996
-
- Vamvakas EC, Pineda AA. Meta‐analysis of clinical studies of the efficacy of granulocyte transfusions in the treatment of bacterial sepsis. Journal of Clinical Apheresis 1996;11(1):1‐9. - PubMed
Vamvakas 1997
-
- Vamvakas EC, Pineda AA. Determinants of the efficacy of prophylactic granulocyte transfusions: a meta‐analysis. Journal of Clinical Apheresis 1997;12(2):74‐81. - PubMed
WHO 1992
-
- World Health Organization. International statistical classification of diseases and related health problems. 10th Edition. Vol. 1, Geneva: World Health Organization, 1992.
References to other published versions of this review
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

