Distinct Roles of Type I and Type III Interferons in Intestinal Immunity to Homologous and Heterologous Rotavirus Infections
- PMID: 27128797
- PMCID: PMC4851417
- DOI: 10.1371/journal.ppat.1005600
Distinct Roles of Type I and Type III Interferons in Intestinal Immunity to Homologous and Heterologous Rotavirus Infections
Erratum in
-
Correction: Distinct Roles of Type I and Type III Interferons in Intestinal Immunity to Homologous and Heterologous Rotavirus Infections.PLoS Pathog. 2016 Jun 15;12(6):e1005726. doi: 10.1371/journal.ppat.1005726. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27304875 Free PMC article.
Abstract
Type I (IFN-α/β) and type III (IFN-λ) interferons (IFNs) exert shared antiviral activities through distinct receptors. However, their relative importance for antiviral protection of different organ systems against specific viruses remains to be fully explored. We used mouse strains deficient in type-specific IFN signaling, STAT1 and Rag2 to dissect distinct and overlapping contributions of type I and type III IFNs to protection against homologous murine (EW-RV strain) and heterologous (non-murine) simian (RRV strain) rotavirus infections in suckling mice. Experiments demonstrated that murine EW-RV is insensitive to the action of both types of IFNs, and that timely viral clearance depends upon adaptive immune responses. In contrast, both type I and type III IFNs can control replication of the heterologous simian RRV in the gastrointestinal (GI) tract, and they cooperate to limit extra-intestinal simian RRV replication. Surprisingly, intestinal epithelial cells were sensitive to both IFN types in neonatal mice, although their responsiveness to type I, but not type III IFNs, diminished in adult mice, revealing an unexpected age-dependent change in specific contribution of type I versus type III IFNs to antiviral defenses in the GI tract. Transcriptional analysis revealed that intestinal antiviral responses to RV are triggered through either type of IFN receptor, and are greatly diminished when receptors for both IFN types are lacking. These results also demonstrate a murine host-specific resistance to IFN-mediated antiviral effects by murine EW-RV, but the retention of host efficacy through the cooperative action by type I and type III IFNs in restricting heterologous simian RRV growth and systemic replication in suckling mice. Collectively, our findings revealed a well-orchestrated spatial and temporal tuning of innate antiviral responses in the intestinal tract where two types of IFNs through distinct patterns of their expression and distinct but overlapping sets of target cells coordinately regulate antiviral defenses against heterologous or homologous rotaviruses with substantially different effectiveness.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: SVK is an inventor on patents and patent applications related to IFN-λs, which have been licensed for commercial development.
Figures
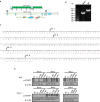
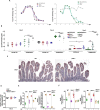
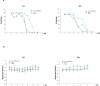
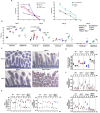
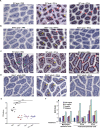
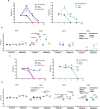

References
-
- Ramig RF. Pathogenesis of intestinal and systemic rotavirus infection. J Virol. 2004;78(19):10213–20. Epub 2004/09/16. doi: 10.1128/JVI.78.19.10213–10220.2004 78/19/10213 [pii]. . - DOI - PMC - PubMed
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(2):136–41. Epub 2011/10/28. doi: S1473-3099(11)70253-5 [pii] 10.1016/S1473-3099(11)70253-5 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

