Hepatitis C Virus Selectively Alters the Intracellular Localization of Desmosterol
- PMID: 27128812
- PMCID: PMC5025300
- DOI: 10.1021/acschembio.6b00324
Hepatitis C Virus Selectively Alters the Intracellular Localization of Desmosterol
Abstract
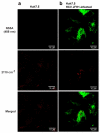
Hepatitis C virus (HCV) increases intracellular desmosterol without affecting the steady-state abundance of other sterols, and the antiviral activity of inhibitors of desmosterol synthesis is suppressed by the addition of exogenous desmosterol. These observations suggest a model in which desmosterol has a specific function, direct or indirect, in HCV replication and that HCV alters desmosterol homeostasis to promote viral replication. Here, we use stimulated Raman scattering (SRS) microscopy in combination with isotopically labeled sterols to show that HCV causes desmosterol to accumulate in lipid droplets that are closely associated with the viral NS5A protein and that are visually distinct from the broad distribution of desmosterol in mock-infected cells and the more heterogeneous and disperse lipid droplets to which cholesterol traffics. Localization of desmosterol in NS5A-associated lipid droplets suggests that desmosterol may affect HCV replication via a direct mechanism. We anticipate that SRS microscopy and similar approaches can provide much needed tools to study the localization of specific lipid molecules in cellulo and in vivo.
Figures



References
-
- World Health Organization, M. C. Hepatitis C. Jul, 2015.
-
- Clejan S, Bittman R. Distribution and movement of sterols with different side chain structures between the two leaflets of the membrane bilayer of mycoplasma cells. J Biol Chem. 1984;259:449–455. - PubMed
-
- Nelson LD, Johnson AE, London E. How interaction of perfringolysin O with membranes is controlled by sterol structure, lipid structure, and physiological low pH: insights into the origin of perfringolysin O-lipid raft interaction. J Biol Chem. 2008;283:4632–4642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

