Introducing Thermal Wave Transport Analysis (TWTA): A Thermal Technique for Dopamine Detection by Screen-Printed Electrodes Functionalized with Molecularly Imprinted Polymer (MIP) Particles
- PMID: 27128891
- PMCID: PMC6272947
- DOI: 10.3390/molecules21050552
Introducing Thermal Wave Transport Analysis (TWTA): A Thermal Technique for Dopamine Detection by Screen-Printed Electrodes Functionalized with Molecularly Imprinted Polymer (MIP) Particles
Abstract
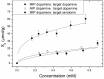
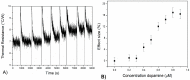
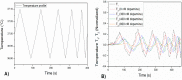
A novel procedure is developed for producing bulk modified Molecularly Imprinted Polymer (MIP) screen-printed electrodes (SPEs), which involves the direct mixing of the polymer particles within the screen-printed ink. This allowed reduction of the sample preparation time from 45 min to 1 min, and resulted in higher reproducibility of the electrodes. The samples are measured with a novel detection method, namely, thermal wave transport analysis (TWTA), relying on the analysis of thermal waves through a functional interface. As a first proof-of-principle, MIPs for dopamine are developed and successfully incorporated within a bulk modified MIP SPE. The detection limits of dopamine within buffer solutions for the MIP SPEs are determined via three independent techniques. With cyclic voltammetry this was determined to be 4.7 × 10(-6) M, whereas by using the heat-transfer method (HTM) 0.35 × 10(-6) M was obtained, and with the novel TWTA concept 0.26 × 10(-6) M is possible. This TWTA technique is measured simultaneously with HTM and has the benefits of reducing measurement time to less than 5 min and increasing effect size by nearly a factor of two. The two thermal methods are able to enhance dopamine detection by one order of magnitude compared to the electrochemical method. In previous research, it was not possible to measure neurotransmitters in complex samples with HTM, but with the improved signal-to-noise of TWTA for the first time, spiked dopamine concentrations were determined in a relevant food sample. In summary, novel concepts are presented for both the sensor functionalization side by employing screen-printing technology, and on the sensing side, the novel TWTA thermal technique is reported. The developed bio-sensing platform is cost-effective and suitable for mass-production due to the nature of screen-printing technology, which makes it very interesting for neurotransmitter detection in clinical diagnostic applications.
Keywords: biomimetic sensors; cyclic voltammetry; heat-transfer method (HTM); molecularly imprinted polymers (MIPs); neurotransmitters; screen-printing technology; thermal wave transport analysis (TWTA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Matsui J., Nicholls I.A., Takeuchi T., Mosbach K., Karube I. Molecularly-imprinted polymeric logic gates selective for predetermined chemical input species. Anal. Chim. Acta. 1996;10:71–77. doi: 10.1016/S0003-2670(96)00356-X. - DOI
-
- Yano K., Karube I. Molecularly imprinted polymers for biosensor applications. TrAC Trends Anal. Chem. 1999;18:199–204. doi: 10.1016/S0165-9936(98)00119-8. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

