Chemical Constituents of Malaysian U. cordata var. ferruginea and Their in Vitro α-Glucosidase Inhibitory Activities
- PMID: 27128898
- PMCID: PMC6274048
- DOI: 10.3390/molecules21050525
Chemical Constituents of Malaysian U. cordata var. ferruginea and Their in Vitro α-Glucosidase Inhibitory Activities
Abstract
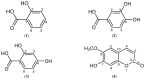
Continuing our interest in the Uncaria genus, the phytochemistry and the in-vitro α-glucosidase inhibitory activities of Malaysian Uncaria cordata var. ferruginea were investigated. The phytochemical study of this plant, which employed various chromatographic techniques including recycling preparative HPLC, led to the isolation of ten compounds with diverse structures comprising three phenolic acids, two coumarins, three flavonoids, a terpene and an iridoid glycoside. These constituents were identified as 2-hydroxybenzoic acid or salicylic acid (1), 2,4-dihydroxybenzoic acid (2), 3,4-dihydroxybenzoic acid (3), scopoletin or 7-hydroxy-6-methoxy-coumarin (4), 3,4-dihydroxy-7-methoxycoumarin (5), quercetin (6), kaempferol (7), taxifolin (8), loganin (9) and β-sitosterol (10). Structure elucidation of the compounds was accomplished with the aid of 1D and 2D Nuclear Magnetic Resonance (NMR) spectral data and Ultraviolet-Visible (UV-Vis), Fourier Transform Infrared (FTIR) spectroscopy and mass spectrometry (MS). In the α-glucosidase inhibitory assay, the crude methanolic extract of the stems of the plant and its acetone fraction exhibited strong α-glucosidase inhibition activity of 87.7% and 89.2%, respectively, while its DCM fraction exhibited only moderate inhibition (75.3%) at a concentration of 1 mg/mL. The IC50 values of both fractions were found to be significantly lower than the standard acarbose suggesting the presence of potential α-glucosidase inhibitors. Selected compounds isolated from the active fractions were then subjected to α-glucosidase assay in which 2,4-dihydroxybenzoic acid and quercetin showed strong inhibitory effects against the enzyme with IC50 values of 549 and 556 μg/mL compared to acarbose (IC50 580 μg/mL) while loganin and scopoletin only showed weak α-glucosidase inhibition of 44.9% and 34.5%, respectively. This is the first report of the isolation of 2-hydroxybenzoic acid, 2,4-dihydroxybenzoic acid and loganin from the genus and the first report of the α-glucosidase inhibitory potential of 2,4-dihydroxybenzoic acid.
Keywords: 2,4-dihydroxybenzoic acid; 2-hydroxybenzoic acid; Uncaria cordata; loganin; phytochemistry; α-glucosidase inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Salim F., Ahmad R. Isopteropodic acid from Malaysian Uncaria longiflora var. pteropoda. World Appl. Sci. J. 2010;10:1334–1337.
-
- Andre N., Wang X.M., He Y., Pan G., Kojo A., Liu Y. A review of the occurrence of non-alkaloid constituents in Uncaria species and their structure-activity relationships. Am. J. Biomed. Life Sci. 2013;1:79–98. doi: 10.11648/j.ajbls.20130104.13. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

