Metabolic Inflammation-Differential Modulation by Dietary Constituents
- PMID: 27128935
- PMCID: PMC4882660
- DOI: 10.3390/nu8050247
Metabolic Inflammation-Differential Modulation by Dietary Constituents
Abstract
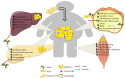
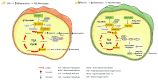
Obesity arises from a sustained positive energy balance which triggers a pro-inflammatory response, a key contributor to metabolic diseases such as T2D. Recent studies, focused on the emerging area of metabolic-inflammation, highlight that specific metabolites can modulate the functional nature and inflammatory phenotype of immune cells. In obesity, expanding adipose tissue attracts immune cells, creating an inflammatory environment within this fatty acid storage organ. Resident immune cells undergo both a pro-inflammatory and metabolic switch in their function. Inflammatory mediators, such as TNF-α and IL-1β, are induced by saturated fatty acids and disrupt insulin signaling. Conversely, monounsaturated and polyunsaturated fatty acids do not interrupt metabolism and inflammation to the same extent. AMPK links inflammation, metabolism and T2D, with roles to play in all and is influenced negatively by obesity. Lipid spillover results in hepatic lipotoxicity and steatosis. Also in skeletal muscle, excessive FFA can impede insulin's action and promote inflammation. Ectopic fat can also affect pancreatic β-cell function, thereby contributing to insulin resistance. Therapeutics, lifestyle changes, supplements and dietary manipulation are all possible avenues to combat metabolic inflammation and the subsequent insulin resistant state which will be explored in the current review.
Keywords: adipose tissue; diet; fatty acids; insulin resistance; liver; metabolic-inflammation; muscle; nutrition; pancreas.
Figures

References
-
- Servier Medical Art. [(accessed on 15 January 2016)]. Available online: http://www.servier.fr/servier-medical-art.
-
- Stienstra R., Joosten L.A.B., Koenen T., van Tits B., van Diepen J.A., van den Berg S.A.A., Rensen P.C.N., Voshol P.J., Fantuzzi G., Hijmans A., et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

