CTNNA1 hypermethylation, a frequent event in acute myeloid leukemia, is independently associated with an adverse outcome
- PMID: 27129146
- PMCID: PMC5058770
- DOI: 10.18632/oncotarget.8962
CTNNA1 hypermethylation, a frequent event in acute myeloid leukemia, is independently associated with an adverse outcome
Abstract
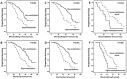
The aim of this study is to evaluate the frequency of CTNNA1 hypermethylation in acute myeloid leukemia (AML) patients in an attempt to improve molecular prognostic model. CTNNA1 promoter methylation levels in 319 newly diagnosed AML patients were detected using quantitative methylation-specific polymerase chain reaction (qMS-PCR). Furthermore, hematological characteristics, cytogenetic abnormalities, and genetic mutation status were analyzed, followed by assessment of clinical impact. Our findings demonstrated that CTNNA1 hypermethylation was observed in 25% AML patients. Hypermethylation of the CTNNA1 promoter was associated with unfavorable karyotype, and also possessed the higher frequency of coexisting with ASXL1 and RUNX1 mutations. Patients with CTNNA1 hypermethylation exhibited the shorter relapse-free survival (RFS) and overall survival (OS) in the whole AML and non-M3 AML patients. Moreover, patients with the higher methylation levels had more aggressive course than those with relative lower levels. In multivariate analyses, CTNNA1 hypermethylation was an independent factor predicting for poor RFS, but not for OS. In conclusion, CTNNA1 hypermethylation may be a reliable factor for improving prognostic molecular model for AML.
Keywords: CTNNA1; acute myeloid leukemia; clinical impact; hypermethylation; survival.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures



Similar articles
-
Methylation of CTNNA1 promoter: frequent but not an adverse prognostic factor in acute myeloid leukemia.Leuk Res. 2014 May;38(5):613-8. doi: 10.1016/j.leukres.2014.03.002. Epub 2014 Mar 11. Leuk Res. 2014. PMID: 24685333
-
CHFR hypermethylation, a frequent event in acute myeloid leukemia, is independently associated with an adverse outcome.Genes Chromosomes Cancer. 2016 Feb;55(2):158-68. doi: 10.1002/gcc.22322. Epub 2015 Nov 6. Genes Chromosomes Cancer. 2016. PMID: 26542416
-
Hypermethylation of the GATA binding protein 4 (GATA4) promoter in Chinese pediatric acute myeloid leukemia.BMC Cancer. 2015 Oct 21;15:756. doi: 10.1186/s12885-015-1760-5. BMC Cancer. 2015. PMID: 26490736 Free PMC article.
-
Trisomy 8 in acute myeloid leukemia.Expert Rev Hematol. 2019 Nov;12(11):947-958. doi: 10.1080/17474086.2019.1657400. Epub 2019 Sep 26. Expert Rev Hematol. 2019. PMID: 31422708 Review.
-
Prognostic Value of RUNX1 Mutations in AML: A Meta-Analysis.Asian Pac J Cancer Prev. 2018 Feb 26;19(2):325-329. doi: 10.22034/APJCP.2018.19.2.325. Asian Pac J Cancer Prev. 2018. PMID: 29479958 Free PMC article. Review.
Cited by
-
α-E-Catenin (CTNNA1) Inhibits Cell Proliferation, Invasion and EMT of Bladder Cancer.Cancer Manag Res. 2020 Dec 14;12:12747-12758. doi: 10.2147/CMAR.S259269. eCollection 2020. Cancer Manag Res. 2020. PMID: 33364826 Free PMC article.
-
RASSF1A hypermethylation is associated with ASXL1 mutation and indicates an adverse outcome in non-M3 acute myeloid leukemia.Onco Targets Ther. 2017 Aug 22;10:4143-4151. doi: 10.2147/OTT.S142528. eCollection 2017. Onco Targets Ther. 2017. PMID: 28860824 Free PMC article.
-
The Role of CTNNA1 in Malignancies: An Updated Review.J Cancer. 2023 Jan 1;14(2):219-230. doi: 10.7150/jca.79236. eCollection 2023. J Cancer. 2023. PMID: 36741258 Free PMC article. Review.
-
Targeting β-catenin in acute myeloid leukaemia: past, present, and future perspectives.Biosci Rep. 2022 Apr 29;42(4):BSR20211841. doi: 10.1042/BSR20211841. Biosci Rep. 2022. PMID: 35352805 Free PMC article. Review.
References
-
- Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381:484–495. - PubMed
-
- Chung SS. Genetic mutations in acute myeloid leukemia that influence clinical decisions. Curr Opin Hematol. 2014;21:87–94. - PubMed
-
- Yang J, Schiffer CA. Genetic biomarkers in acute myeloid leukemia: will the promise of improving treatment outcomes be realized? Expert Rev Hematol. 2012;5:395–407. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

