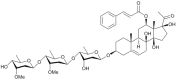
Asclepiasterol, a novel C21 steroidal glycoside derived from Asclepias curassavica, reverses tumor multidrug resistance by down-regulating P-glycoprotein expression
- PMID: 27129170
- PMCID: PMC5058771
- DOI: 10.18632/oncotarget.8965
Asclepiasterol, a novel C21 steroidal glycoside derived from Asclepias curassavica, reverses tumor multidrug resistance by down-regulating P-glycoprotein expression
Abstract
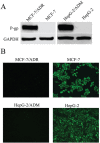
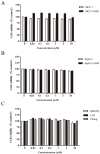
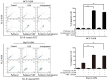
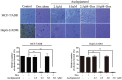
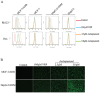
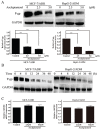
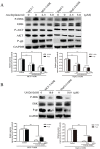
Multidrug resistance (MDR) mediated by P-glycoprotein (P-gp) is a major cause of cancer therapy failure. In this study, we identified a novel C21 steroidal glycoside, asclepiasterol, capable of reversing P-gp-mediated MDR. Asclepiasterol (2.5 and 5.0μM) enhanced the cytotoxity of P-gp substrate anticancer drugs in MCF-7/ADR and HepG-2/ADM cells. MDR cells were more responsive to paclitaxel in the presence of asclepiasterol, and colony formation of MDR cells was only reduced upon treatment with a combination of asclepiasterol and doxorubicin. Consistent with these findings, asclepiasterol treatment increased the intracellular accumulation of doxorubicin and rhodamine 123 (Rh123) in MDR cells. Asclepiasterol decreased expression of P-gp protein without stimulating or suppressing MDR1 mRNA levels. Asclepiasterol-mediated P-gp suppression caused inhibition of ERK1/2 phosphorylation in two MDR cell types, and EGF, an activator of the MAPK/ERK pathway, reversed the P-gp down-regulation, implicating the MAPK/ERK pathway in asclepiasterol-mediated P-gp down-regulation. These results suggest that asclepiasterol could be developed as a modulator for reversing P-gp-mediated MDR in P-gp-overexpressing cancer variants.
Keywords: P-glycoprotein; asclepiasterol; multidrug resistance (MDR); phosphorylation of ERK1/2 (P-ERK); steroid.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures

Similar articles
-
The effects of ultrasound exposure on P-glycoprotein-mediated multidrug resistance in vitro and in vivo.J Exp Clin Cancer Res. 2018 Sep 19;37(1):232. doi: 10.1186/s13046-018-0900-6. J Exp Clin Cancer Res. 2018. PMID: 30231924 Free PMC article.
-
Reversal of P-glycoprotein-mediated multidrug resistance is induced by saikosaponin D in breast cancer MCF-7/adriamycin cells.Pathol Res Pract. 2017 Jul;213(7):848-853. doi: 10.1016/j.prp.2017.01.022. Epub 2017 Feb 3. Pathol Res Pract. 2017. PMID: 28554760
-
Dasatinib reverses the multidrug resistance of breast cancer MCF-7 cells to doxorubicin by downregulating P-gp expression via inhibiting the activation of ERK signaling pathway.Cancer Biol Ther. 2015;16(1):106-14. doi: 10.4161/15384047.2014.987062. Cancer Biol Ther. 2015. PMID: 25482933 Free PMC article.
-
Reversal of multidrug resistance of tumor cells.Anticancer Res. 2000 Nov-Dec;20(6B):4261-74. Anticancer Res. 2000. PMID: 11205256 Review.
-
Interplay between P-Glycoprotein Expression and Resistance to Endoplasmic Reticulum Stressors.Molecules. 2018 Feb 6;23(2):337. doi: 10.3390/molecules23020337. Molecules. 2018. PMID: 29415493 Free PMC article. Review.
Cited by
-
The effects of ultrasound exposure on P-glycoprotein-mediated multidrug resistance in vitro and in vivo.J Exp Clin Cancer Res. 2018 Sep 19;37(1):232. doi: 10.1186/s13046-018-0900-6. J Exp Clin Cancer Res. 2018. PMID: 30231924 Free PMC article.
-
Antiproliferative effect of ZSTK474 alone or in combination with chemotherapeutic drugs on HL60 and HL60/ADR cells.Oncotarget. 2017 Jun 13;8(24):39064-39076. doi: 10.18632/oncotarget.16589. Oncotarget. 2017. PMID: 28388564 Free PMC article.
-
Tomentodione M sensitizes multidrug resistant cancer cells by decreasing P-glycoprotein via inhibition of p38 MAPK signaling.Oncotarget. 2017 Oct 19;8(60):101965-101983. doi: 10.18632/oncotarget.21949. eCollection 2017 Nov 24. Oncotarget. 2017. PMID: 29254218 Free PMC article.
-
Genome and Tissue-Specific Transcriptome of the Tropical Milkweed (Asclepias curassavica).Plant Direct. 2025 Mar 18;9(3):e70031. doi: 10.1002/pld3.70031. eCollection 2025 Mar. Plant Direct. 2025. PMID: 40103632 Free PMC article.
-
Lung resistance-related protein (LRP) predicts favorable therapeutic outcome in Acute Myeloid Leukemia.Sci Rep. 2019 Jan 23;9(1):378. doi: 10.1038/s41598-018-36780-8. Sci Rep. 2019. PMID: 30674943 Free PMC article. Clinical Trial.
References
-
- Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. - PubMed
-
- Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annual review of biochemistry. 2002;71:537–592. - PubMed
-
- Shen D, Pastan I, Gottesman MM. Cross-resistance to methotrexate and metals in human cisplatin-resistant cell lines results from a pleiotropic defect in accumulation of these compounds associated with reduced plasma membrane binding proteins. Cancer research. 1998;58:268–275. - PubMed
-
- Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome research. 2001;11:1156–1166. - PubMed
-
- Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

