Tighter Control by Chymotrypsin C (CTRC) Explains Lack of Association between Human Anionic Trypsinogen and Hereditary Pancreatitis
- PMID: 27129265
- PMCID: PMC4933207
- DOI: 10.1074/jbc.M116.725374
Tighter Control by Chymotrypsin C (CTRC) Explains Lack of Association between Human Anionic Trypsinogen and Hereditary Pancreatitis
Abstract
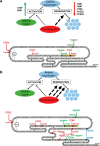
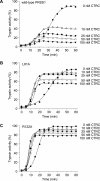
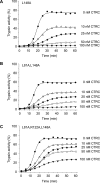
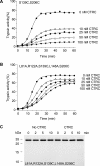
The human pancreas expresses two major trypsinogen isoforms, cationic trypsinogen (PRSS1) and anionic trypsinogen (PRSS2). Mutations in PRSS1 cause hereditary pancreatitis by altering cleavage of regulatory nick sites by chymotrypsin C (CTRC) resulting in reduced trypsinogen degradation and increased autoactivation. Despite 90% identity with PRSS1 and a strong propensity for autoactivation, mutations in PRSS2 are not found in hereditary pancreatitis suggesting that activation of this isoform is more tightly regulated. Here, we demonstrated that CTRC promoted degradation and thereby markedly suppressed autoactivation of human anionic trypsinogen more effectively than previously observed with cationic trypsinogen. Increased sensitivity of anionic trypsinogen to CTRC-mediated degradation was due to an additional cleavage site at Leu-148 in the autolysis loop and the lack of the conserved Cys-139-Cys-206 disulfide bond. Significant stabilization of anionic trypsinogen against degradation was achieved by simultaneous mutations of CTRC cleavage sites Leu-81 and Leu-148, autolytic cleavage site Arg-122, and restoration of the missing disulfide bridge. This stands in stark contrast to cationic trypsinogen where single mutations of either Leu-81 or Arg-122 resulted in almost complete resistance to CTRC-mediated degradation. Finally, processing of the trypsinogen activation peptide at Phe-18 by CTRC inhibited autoactivation of anionic trypsinogen, although cationic trypsinogen was strongly stimulated. Taken together, the observations indicate that human anionic trypsinogen is controlled by CTRC in a manner that individual natural mutations are unlikely to increase stability enough to promote intra-pancreatic activation. This unique biochemical property of anionic trypsinogen explains the lack of association of PRSS2 mutations with hereditary pancreatitis.
Keywords: chymotrypsin; chymotrypsin C; pancreas; serine protease; trypsin; trypsinogen.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Chen J.-M., and Ferec C. (2000) Genes, cloned cDNAs, and proteins of human trypsinogens and pancreatitis-associated cationic trypsinogen mutations. Pancreas 21, 57–62 - PubMed
-
- Guy O., Lombardo D., Bartelt D. C., Amic J., and Figarella C. (1978) Two human trypsinogens. Purification, molecular properties, and N-terminal sequences. Biochemistry 17, 1669–1675 - PubMed
-
- Scheele G., Bartelt D., and Bieger W. (1981) Characterization of human exocrine pancreatic proteins by two-dimensional isoelectric focusing/sodium dodecyl sulfate gel electrophoresis. Gastroenterology 80, 461–473 - PubMed
-
- Rinderknecht H., Stace N. H., and Renner I. G. (1985) Effects of chronic alcohol abuse on exocrine pancreatic secretion in man. Dig. Dis. Sci. 30, 65–71 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

