Neurochemical Mediation of Affiliation and Aggression Associated With Pair-Bonding
- PMID: 27129413
- PMCID: PMC4992658
- DOI: 10.1016/j.biopsych.2016.02.013
Neurochemical Mediation of Affiliation and Aggression Associated With Pair-Bonding
Abstract
Background: The neuropeptides vasopressin and corticotropin-releasing factor facilitate, while serotonin inhibits, aggression. How the brain is wired to coordinate interactions between these functionally opposed neurotransmitters to control behavioral states is poorly understood.
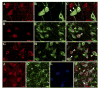
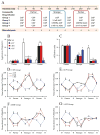
Methods: Pair-bonded male prairie voles (Microtus ochrogaster) were infused with a retrograde tracer, Fluoro-Gold, and tested for affiliation and aggression toward a female partner or novel female subject. Subsequent immunocytochemical experiments examined neuronal activation using Fos and neurochemical/neuroreceptor profiles on brain areas involved in these social behaviors. Finally, a series of behavioral pharmacologic and real-time in vivo brain microdialysis experiments were performed on male prairie voles displaying affiliation or aggression.
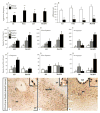
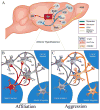
Results: We localized a subpopulation of excitatory vasopressin neurons in the anterior hypothalamus that may gate corticotropin-releasing factor output from the amygdala to the anterior hypothalamus and then the lateral septum to modulate aggression associated with mate guarding. Conversely, we identified a subset of inhibitory serotonergic projection neurons in the dorsal raphe that project to the anterior hypothalamus and may mediate the spatiotemporal release of neuropeptides and their interactions in modulating aggression and affiliation.
Conclusions: Together, this study establishes the medial extended amygdala as a major neural substrate regulating the switch between positive and negative affective states, wherein several neurochemicals converge and interact to coordinate divergent social behaviors.
Keywords: Anterior hypothalamus; Corticotropin-releasing factor; Dopamine; Medial amygdala; Serotonin; Vasopressin.
Published by Elsevier Inc.
Conflict of interest statement
All authors report no biomedical financial interests or potential conflicts of interest.
Figures

Comment in
-
Neurochemicals Drawing the Line Between Love and Hate.Biol Psychiatry. 2017 Feb 1;81(3):177-178. doi: 10.1016/j.biopsych.2016.11.003. Biol Psychiatry. 2017. PMID: 28024706 No abstract available.
References
-
- Luiten PG, Koolhaas JM, de Boer S, Koopmans SJ. The cortico-medial amygdala in the central nervous system organization of agonistic behavior. Brain Res. 1985;332(2):283–97. - PubMed
-
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, et al. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46(4):647–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

