Secreted Frizzled-related Protein 2 (sFRP2) Redirects Non-canonical Wnt Signaling from Fz7 to Ror2 during Vertebrate Gastrulation
- PMID: 27129770
- PMCID: PMC4919455
- DOI: 10.1074/jbc.M116.733766
Secreted Frizzled-related Protein 2 (sFRP2) Redirects Non-canonical Wnt Signaling from Fz7 to Ror2 during Vertebrate Gastrulation
Abstract
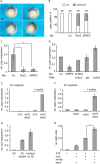
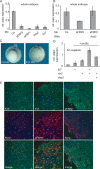
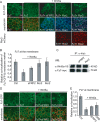
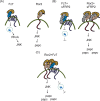
Convergent extension movements during vertebrate gastrulation require a balanced activity of non-canonical Wnt signaling pathways, but the factors regulating this interplay on the molecular level are poorly characterized. Here we show that sFRP2, a member of the secreted frizzled-related protein (sFRP) family, is required for morphogenesis and papc expression during Xenopus gastrulation. We further provide evidence that sFRP2 redirects non-canonical Wnt signaling from Frizzled 7 (Fz7) to the receptor tyrosine kinase-like orphan receptor 2 (Ror2). During this process, sFRP2 promotes Ror2 signal transduction by stabilizing Wnt5a-Ror2 complexes at the membrane, whereas it inhibits Fz7 signaling, probably by blocking Fz7 receptor endocytosis. The cysteine-rich domain of sFRP2 is sufficient for Ror2 activation, and related sFRPs can substitute for this function. Notably, direct interaction of the two receptors via their cysteine-rich domains also promotes Ror2-mediated papc expression but inhibits Fz7 signaling. We propose that sFRPs can act as a molecular switch, channeling the signal input for different non-canonical Wnt pathways during vertebrate gastrulation.
Keywords: Fz7; Ror2; Wnt signaling; Xenopus; development; embryo; papc; sFRP2; zebrafish.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Kumar S., Žigman M., Patel T. R., Trageser B., Gross J. C., Rahm K., Boutros M., Gradl D., Steinbeisser H., Holstein T., Stetefeld J., and Özbek S. (2014) Molecular dissection of Wnt3a-Frizzled8 interaction reveals essential and modulatory determinants of Wnt signaling activity. BMC Biol. 12, 44. - PMC - PubMed
-
- Dann C. E., Hsieh J. C., Rattner A., Sharma D., Nathans J., and Leahy D. J. (2001) Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 412, 86–90 - PubMed
-
- Niehrs C. (2012) The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 13, 767–779 - PubMed
-
- Malinauskas T., and Jones E. Y. (2014) Extracellular modulators of Wnt signalling. Curr. Opin. Struct. Biol. 29, 77–84 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

