Limits of Versatility of Versatile Peroxidase
- PMID: 27129968
- PMCID: PMC4959207
- DOI: 10.1128/AEM.00743-16
Limits of Versatility of Versatile Peroxidase
Abstract
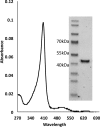
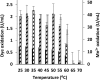
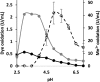
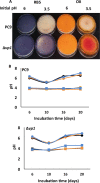
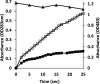
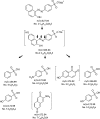
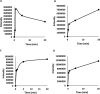
Although Mn(2+) is the most abundant substrate of versatile peroxidases (VPs), repression of Pleurotus ostreatus vp1 expression occurred in Mn(2+)-sufficient medium. This seems to be a biological contradiction. The aim of this study was to explore the mechanism of direct oxidation by VP1 under Mn(2+)-deficient conditions, as it was found to be the predominant enzyme during fungal growth in the presence of synthetic and natural substrates. The native VP1 was purified and characterized using three substrates, Mn(2+), Orange II (OII), and Reactive Black 5 (RB5), each oxidized by a different active site in the enzyme. While the pH optimum for Mn(2+) oxidation is 5, the optimum pH for direct oxidation of both dyes was found to be 3. Indeed, effective in vivo decolorization occurred in media without addition of Mn(2+) only under acidic conditions. We have determined that Mn(2+) inhibits in vitro the direct oxidation of both OII and RB5 while RB5 stabilizes both Mn(2+) and OII oxidation. Furthermore, OII was found to inhibit the oxidation of both Mn(2+) and RB5. In addition, we could demonstrate that VP1 can cleave OII in two different modes. Under Mn(2+)-mediated oxidation conditions, VP1 was able to cleave the azo bond only in asymmetric mode, while under the optimum conditions for direct oxidation (absence of Mn(2+) at pH 3) both symmetric and asymmetric cleavages occurred. We concluded that the oxidation mechanism of aromatic compounds by VP1 is controlled by Mn(2+) and pH levels both in the growth medium and in the reaction mixture.
Importance: VP1 is a member of the ligninolytic heme peroxidase gene family of the white rot fungus Pleurotus ostreatus and plays a fundamental role in biodegradation. This enzyme exhibits a versatile nature, as it can oxidize different substrates under altered environmental conditions. VPs are highly interesting enzymes due to the fact that they contain unique active sites that are responsible for direct oxidation of various aromatic compounds, including lignin, in addition to the well-known Mn(2+) binding active site. This study demonstrates the limits of versatility of P. ostreatus VP1, which harbors multiple active sites, exhibiting a broad range of enzymatic activities, but they perform differently under distinct conditions. The versatility of P. ostreatus and its enzymes is an advantageous factor in the fungal ability to adapt to changing environments. This trait expands the possibilities for the potential utilization of P. ostreatus and other white rot fungi.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Fernandez-Fueyo E, Ruiz-Duenas FJ, Jesus Martinez M, Romero A, Hammel KE, Javier Medrano F, Martinez AT. 2014. Ligninolytic peroxidase genes in the oyster mushroom genome: heterologous expression, molecular structure, catalytic and stability properties, and lignin-degrading ability. Biotechnol Biofuels 7:2. doi:10.1186/1754-6834-7-2. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

