Recombinant human erythropoietin improves neurological outcomes in very preterm infants
- PMID: 27130143
- PMCID: PMC5084793
- DOI: 10.1002/ana.24677
Recombinant human erythropoietin improves neurological outcomes in very preterm infants
Abstract
Objective: To evaluate the efficacy and safety of repeated low-dose human recombinant erythropoietin (rhEPO) in the improvement of neurological outcomes in very preterm infants.
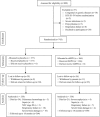
Methods: A total of 800 infants of ≤32-week gestational age who had been in an intensive care unit within 72 hours after birth were included in the trial between January 2009 and June 2013. Preterm infants were randomly assigned to receive rhEPO (500IU/kg; n = 366) or placebo (n = 377) intravenously within 72 hours after birth and then once every other day for 2 weeks. The primary outcome was death or moderate to severe neurological disability assessed at 18 months of corrected age.
Results: Death and moderate/severe neurological disability occurred in 91 of 338 very preterm infants (26.9%) in the placebo group and in 43 of 330 very preterm infants (13.0%) in the rhEPO treatment group (relative risk [RR] = 0.40, 95% confidence interval [CI] = 0.27-0.59, p < 0.001) at 18 months of corrected age. The rate of moderate/severe neurological disability in the rhEPO group (22 of 309, 7.1%) was significantly lower compared to the placebo group (57 of 304, 18.8%; RR = 0.32, 95% CI = 0.19-0.55, p < 0.001), and no excess adverse events were observed.
Interpretation: Repeated low-dose rhEPO treatment reduced the risk of long-term neurological disability in very preterm infants with no obvious adverse effects. Ann Neurol 2016;80:24-34.
© 2016 The Authors. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures
Comment in
-
Erythropoietin for Neuroprotection in preterm infants.Ann Neurol. 2016 Dec;80(6):952. doi: 10.1002/ana.24787. Epub 2016 Oct 19. Ann Neurol. 2016. PMID: 27757982 No abstract available.
References
-
- Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379:2151–2161. - PubMed
-
- Serenius F, Kallen K, Blennow M, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA 2013;309:1810–1820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

