Mechanical Stretching Promotes Skin Tissue Regeneration via Enhancing Mesenchymal Stem Cell Homing and Transdifferentiation
- PMID: 27130223
- PMCID: PMC4922851
- DOI: 10.5966/sctm.2015-0274
Mechanical Stretching Promotes Skin Tissue Regeneration via Enhancing Mesenchymal Stem Cell Homing and Transdifferentiation
Abstract
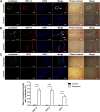
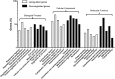
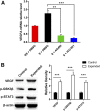
Skin tissue expansion is a clinical procedure for skin regeneration to reconstruct cutaneous defects that can be accompanied by severe complications. The transplantation of mesenchymal stem cells (MSCs) has been proven effective in promoting skin expansion and helping to ameliorate complications; however, systematic understanding of its mechanism remains unclear. MSCs from luciferase-Tg Lewis rats were intravenously transplanted into a rat tissue expansion model to identify homing and transdifferentiation. To clarify underlying mechanisms, a systematic approach was used to identify the differentially expressed genes between mechanically stretched human MSCs and controls. The biological significance of these changes was analyzed through bioinformatic methods. We further investigated genes and pathways of interest to disclose their potential role in mechanical stretching-induced skin regeneration. Cross sections of skin samples from the expanded group showed significantly more luciferase(+) and stromal cell-derived factor 1α (SDF-1α)(+), luciferase(+)keratin 14(+), and luciferase(+)CD31(+) cells than the control group, indicating MSC transdifferentiation into epidermal basal cells and endothelial cells after SDF-1α-mediated homing. Microarray analysis suggested upregulation of genes related to hypoxia, vascularization, and cell proliferation in the stretched human MSCs. Further investigation showed that the homing of MSCs was blocked by short interfering RNA targeted against matrix metalloproteinase 2, and that mechanical stretching-induced vascular endothelial growth factor A upregulation was related to the Janus kinase/signal transducer and activator of transcription (Jak-STAT) and Wnt signaling pathways. This study determines that mechanical stretching might promote skin regeneration by upregulating MSC expression of genes related to hypoxia, vascularization, and cell proliferation; enhancing transplanted MSC homing to the expanded skin; and transdifferentiation into epidermal basal cells and endothelial cells.
Significance: Skin tissue expansion is a clinical procedure for skin regeneration to cover cutaneous defects that can be accompanied by severe complications. The transplantation of mesenchymal stem cells (MSCs) has been proven effective in promoting skin expansion and ameliorating complications. This study, which sought to provide a systematic understanding of the mechanism, determined that mechanical stretching could upregulate MSC expression of genes related to hypoxia, vascularization, and cell proliferation; enhance transplanted MSC homing to the expanded skin tissue; and promote their transdifferentiation into epidermal basal cells and endothelial cells.
Keywords: Mechanical stretching; Mesenchymal stem cell; Skin regeneration; Stem cell therapy; Tissue expansion.
©AlphaMed Press.
Figures



Similar articles
-
Intravenous transplantation of bone marrow mesenchymal stem cells could effectively promote vascularization and skin regeneration in mechanically stretched skin.Br J Dermatol. 2015;172(5):1278-85. doi: 10.1111/bjd.13251. Epub 2015 Jan 18. Br J Dermatol. 2015. PMID: 25041452
-
Mechanical stretch upregulates SDF-1α in skin tissue and induces migration of circulating bone marrow-derived stem cells into the expanded skin.Stem Cells. 2013 Dec;31(12):2703-13. doi: 10.1002/stem.1479. Stem Cells. 2013. PMID: 23836581
-
Gelatin-based 3D conduits for transdifferentiation of mesenchymal stem cells into Schwann cell-like phenotypes.Acta Biomater. 2017 Apr 15;53:293-306. doi: 10.1016/j.actbio.2017.02.018. Epub 2017 Feb 16. Acta Biomater. 2017. PMID: 28213098
-
The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases.J Cell Mol Med. 2015 Mar;19(3):511-20. doi: 10.1111/jcmm.12482. Epub 2014 Dec 23. J Cell Mol Med. 2015. PMID: 25534251 Free PMC article. Review.
-
Mesenchymal stem cell homing: the devil is in the details.Cell Stem Cell. 2009 Mar 6;4(3):206-16. doi: 10.1016/j.stem.2009.02.001. Cell Stem Cell. 2009. PMID: 19265660 Review.
Cited by
-
The Roles of WNT Signaling Pathways in Skin Development and Mechanical-Stretch-Induced Skin Regeneration.Biomolecules. 2023 Nov 24;13(12):1702. doi: 10.3390/biom13121702. Biomolecules. 2023. PMID: 38136575 Free PMC article. Review.
-
Tissue Regeneration from Mechanical Stretching of Cell-Cell Adhesion.Tissue Eng Part C Methods. 2019 Nov;25(11):631-640. doi: 10.1089/ten.TEC.2019.0098. Epub 2019 Sep 25. Tissue Eng Part C Methods. 2019. PMID: 31407627 Free PMC article. Review.
-
Administration of signalling molecules dictates stem cell homing for in situ regeneration.J Cell Mol Med. 2017 Dec;21(12):3162-3177. doi: 10.1111/jcmm.13286. Epub 2017 Aug 2. J Cell Mol Med. 2017. PMID: 28767189 Free PMC article. Review.
-
Bioactive Materials That Promote the Homing of Endogenous Mesenchymal Stem Cells to Improve Wound Healing.Int J Nanomedicine. 2024 Jul 30;19:7751-7773. doi: 10.2147/IJN.S455469. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39099796 Free PMC article. Review.
-
Mesenchymal stem cells: potential application for the treatment of hepatic cirrhosis.Stem Cell Res Ther. 2018 Mar 9;9(1):59. doi: 10.1186/s13287-018-0814-4. Stem Cell Res Ther. 2018. PMID: 29523186 Free PMC article. Review.
References
-
- Neumann CG. The expansion of an area of skin by progressive distention of a subcutaneous balloon; use of the method for securing skin for subtotal reconstruction of the ear. Plast Reconstr Surg (1946) 1957;19:124–130. - PubMed
-
- De Filippo RE, Atala A. Stretch and growth: The molecular and physiologic influences of tissue expansion. Plast Reconstr Surg. 2002;109:2450–2462. - PubMed
-
- Huang X, Qu X, Li Q. Risk factors for complications of tissue expansion: A 20-year systematic review and meta-analysis. Plast Reconstr Surg. 2011;128:787–797. - PubMed
-
- Hallock GG. Safety of clinical overinflation of tissue expanders. Plast Reconstr Surg. 1995;96:153–157. - PubMed
-
- Wickman M. Comparison between rapid and slow tissue expansion in breast reconstruction. Plast Reconstr Surg. 1993;91:663–670. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

