Piperidinyl thiazole isoxazolines: A new series of highly potent, slowly reversible FAAH inhibitors with analgesic properties
- PMID: 27130358
- PMCID: PMC4936272
- DOI: 10.1016/j.bmcl.2016.02.061
Piperidinyl thiazole isoxazolines: A new series of highly potent, slowly reversible FAAH inhibitors with analgesic properties
Abstract
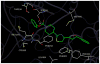
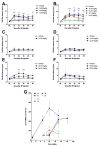
Fatty acid amide hydrolase (FAAH) is a membrane anchored serine hydrolase that has a principle role in the metabolism of the endogenous cannabinoid anandamide. Docking studies using representative FAAH crystal structures revealed that compounds containing a novel piperidinyl thiazole isoxazoline core fit within the ligand binding domains. New potential FAAH inhibitors were designed and synthesized incorporating urea, carbamate, alkyldione and thiourea reactive centers as potential pharmacophores. A small library of candidate compounds (75) was then screened against human FAAH leading to the identification of new carbamate and urea based inhibitors (Ki=pM and nM, respectively). Representative carbamate and urea based chemotypes displayed slow, time dependent inhibition kinetics leading to enzyme inactivation which was slowly reversible. However, evidence indicated that features of the mechanism of inactivation differ between the two pharmacophore types. Selected compounds were also evaluated for analgesic activity in the mouse-tail flick test.
Keywords: Analgesia; Fatty acid amide hydrolase; Inactivation; Inhibition; Piperidinyl thiazole isoxazoline.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical

