Computational neurorehabilitation: modeling plasticity and learning to predict recovery
- PMID: 27130577
- PMCID: PMC4851823
- DOI: 10.1186/s12984-016-0148-3
Computational neurorehabilitation: modeling plasticity and learning to predict recovery
Abstract
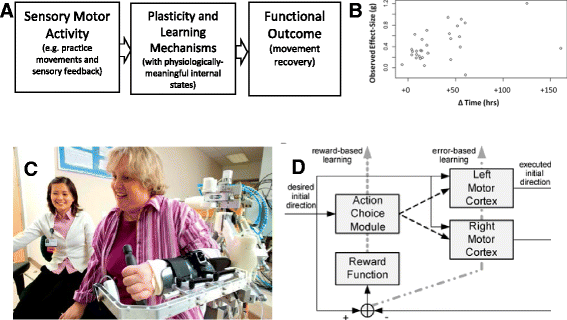
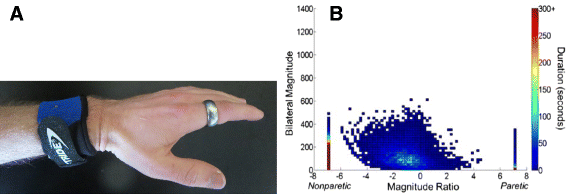
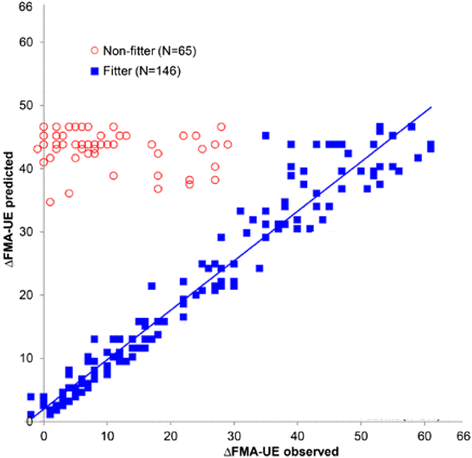
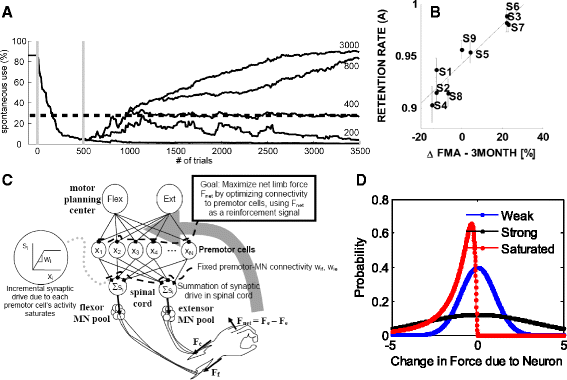
Despite progress in using computational approaches to inform medicine and neuroscience in the last 30 years, there have been few attempts to model the mechanisms underlying sensorimotor rehabilitation. We argue that a fundamental understanding of neurologic recovery, and as a result accurate predictions at the individual level, will be facilitated by developing computational models of the salient neural processes, including plasticity and learning systems of the brain, and integrating them into a context specific to rehabilitation. Here, we therefore discuss Computational Neurorehabilitation, a newly emerging field aimed at modeling plasticity and motor learning to understand and improve movement recovery of individuals with neurologic impairment. We first explain how the emergence of robotics and wearable sensors for rehabilitation is providing data that make development and testing of such models increasingly feasible. We then review key aspects of plasticity and motor learning that such models will incorporate. We proceed by discussing how computational neurorehabilitation models relate to the current benchmark in rehabilitation modeling - regression-based, prognostic modeling. We then critically discuss the first computational neurorehabilitation models, which have primarily focused on modeling rehabilitation of the upper extremity after stroke, and show how even simple models have produced novel ideas for future investigation. Finally, we conclude with key directions for future research, anticipating that soon we will see the emergence of mechanistic models of motor recovery that are informed by clinical imaging results and driven by the actual movement content of rehabilitation therapy as well as wearable sensor-based records of daily activity.
Keywords: Computational modeling; Motor control; Motor learning; Neurorehabilitation; Plasticity; Stroke recovery.
Figures
References
-
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O’Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CMM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245–55. doi: 10.1016/S0140-6736(13)61953-4. - DOI - PMC - PubMed
-
- Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, Billinger SA. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke. 2010;41(10):2402–48. doi: 10.1161/STR.0b013e3181e7512b. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

