A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease
- PMID: 27130705
- PMCID: PMC4946749
- DOI: 10.1093/eurheartj/ehw132
A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease
Abstract
Aims: To evaluate oral doses of the non-steroidal mineralocorticoid receptor antagonist finerenone given for 90 days in patients with worsening heart failure and reduced ejection fraction and chronic kidney disease and/or diabetes mellitus.
Methods and results: Miner Alocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF) was a randomized, double-blind, phase 2b multicentre study (ClinicalTrials.gov: NCT01807221). Of 1286 screened patients, 1066 were randomized. Patients received oral, once-daily finerenone (2.5, 5, 7.5, 10, or 15 mg, uptitrated to 5, 10, 15, 20, or 20 mg, respectively, on Day 30) or eplerenone (25 mg every other day, increased to 25 mg once daily on Day 30, and to 50 mg once daily on Day 60) for 90 days. The primary endpoint was the percentage of individuals with a decrease of >30% in plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) from baseline to Day 90. A key exploratory endpoint was a composite clinical endpoint of death from any cause, cardiovascular hospitalizations, or emergency presentation for worsening HF until Day 90. Mean age ranged from 69.2 to 72.5 years in different treatment groups (standard deviation 9.7-10.6 years). Decreases in NT-proBNP of >30% from baseline occurred in 37.2% of patients in the eplerenone group and 30.9, 32.5, 37.3, 38.8, and 34.2% in the 2.5→5, 5→10, 7.5→15, 10→20, and 15→20 mg finerenone groups, respectively (P = 0.42-0.88). Except for the 2.5→5 mg finerenone group, the composite clinical endpoint occurred numerically less frequently in finerenone-treated patients compared with eplerenone; this difference reached nominal statistical significance in the 10→20 mg group (hazard ratio 0.56, 95% confidence interval, CI, 0.35; 0.90; nominal P = 0.02), despite the fact that this phase 2 study was not designed to detect statistical significant differences. A potassium level increase to ≥5.6 mmol/L at any time point occurred in 4.3% of patients, with a balanced distribution among all treatment groups.
Conclusion: Finerenone was well tolerated and induced a 30% or greater decrease in NT-proBNP levels in a similar proportion of patients to eplerenone. The finding of reduced clinical events in the finerenone 10→20 mg group should be further explored in a large outcomes trial.
Keywords: Finerenone; Mineralocorticoid receptor antagonists; Worsening heart failure.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

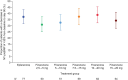
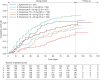

Comment in
-
Finerenone in heart failure: walking a fine line.Eur Heart J. 2016 Jul 14;37(27):2115-7. doi: 10.1093/eurheartj/ehw155. Epub 2016 Apr 29. Eur Heart J. 2016. PMID: 27130706 No abstract available.
-
Non-steroidal aldosterone receptor antagonism: a 'fine' treatment for heart failure patients?Eur J Heart Fail. 2022 Jun;24(6):1006-1008. doi: 10.1002/ejhf.2537. Epub 2022 May 23. Eur J Heart Fail. 2022. PMID: 35560753 No abstract available.
References
-
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709–717. - PubMed
-
- Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11–21.
-
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–1847. - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;128:1810–1852. - PubMed
-
- Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004;351:543–551. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

