Toward Better Genetically Encoded Sensors of Membrane Potential
- PMID: 27130905
- PMCID: PMC4852096
- DOI: 10.1016/j.tins.2016.02.005
Toward Better Genetically Encoded Sensors of Membrane Potential
Abstract
Genetically encoded optical sensors of cell activity are powerful tools that can be targeted to specific cell types. This is especially important in neuroscience because individual brain regions can include a multitude of different cell types. Optical imaging allows for simultaneous recording from numerous neurons or brain regions. Optical signals of membrane potential are useful because membrane potential changes are a direct sign of both synaptic and action potentials. Here we describe recent improvements in the in vitro and in vivo signal size and kinetics of genetically encoded voltage indicators (GEVIs) and discuss their relationship to alternative sensors of neural activity.
Keywords: Ehud Isacoff; Thomas Knopfel; genetically encoded calcium indicators (GECIs); genetically encoded voltage indicators (GEVIs).
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

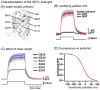

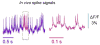


References
-
- Sherrington Sc. Man on His Nature. Cambridge University Press; 1937.
-
- Akemann W, Mutoh H, Perron A, Rossier J, Knöpfel T. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nature Methods. 2010;7:643–649. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

