Avoiding Severe Toxicity From Combined BRAF Inhibitor and Radiation Treatment: Consensus Guidelines from the Eastern Cooperative Oncology Group (ECOG)
- PMID: 27131079
- PMCID: PMC5102246
- DOI: 10.1016/j.ijrobp.2016.01.038
Avoiding Severe Toxicity From Combined BRAF Inhibitor and Radiation Treatment: Consensus Guidelines from the Eastern Cooperative Oncology Group (ECOG)
Erratum in
-
Erratum to: Anker CJ, Grossmann KF, Atkins MB, Suneja G, Tarhini AA, Kirkwood JM. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: Consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys 2016;95:632-646.Int J Radiat Oncol Biol Phys. 2016 Oct 1;96(2):486. doi: 10.1016/j.ijrobp.2016.06.017. Epub 2016 Sep 23. Int J Radiat Oncol Biol Phys. 2016. PMID: 27598818 No abstract available.
Abstract
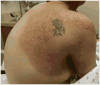
BRAF kinase gene V600 point mutations drive approximately 40% to 50% of all melanomas, and BRAF inhibitors (BRAFi) have been found to significantly improve survival outcomes. Although radiation therapy (RT) provides effective symptom palliation, there is a lack of toxicity and efficacy data when RT is combined with BRAFi, including vemurafenib and dabrafenib. This literature review provides a detailed analysis of potential increased dermatologic, pulmonary, neurologic, hepatic, esophageal, and bowel toxicity from the combination of BRAFi and RT for melanoma patients described in 27 publications. Despite 7 publications noting potential intracranial neurotoxicity, the rates of radionecrosis and hemorrhage from whole brain RT (WBRT), stereotactic radiosurgery (SRS), or both do not appear increased with concurrent or sequential administration of BRAFis. Almost all grade 3 dermatitis reactions occurred when RT and BRAFi were administered concurrently. Painful, disfiguring nondermatitis cutaneous reactions have been described from concurrent or sequential RT and BRAFi administration, which improved with topical steroids and time. Visceral toxicity has been reported with RT and BRAFi, with deaths possibly related to bowel perforation and liver hemorrhage. Increased severity of radiation pneumonitis with BRAFi is rare, but more concerning was a potentially related fatal pulmonary hemorrhage. Conversely, encouraging reports have described patients with leptomeningeal spread and unresectable lymphadenopathy rendered disease free from combined RT and BRAFi. Based on our review, the authors recommend holding BRAFi and/or MEK inhibitors ≥3 days before and after fractionated RT and ≥1 day before and after SRS. No fatal reactions have been described with a dose <4 Gy per fraction, and time off systemic treatment should be minimized. Future prospective data will serve to refine these recommendations.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
none.
Figures

References
-
- Dasgupta T, Haas-Kogan DA, Yang X, et al. Genotype-dependent cooperation of ionizing radiation with BRAF inhibition in BRAF v600e-mutated carcinomas. Invest New Drugs. 2013;31:1136–1141. - PubMed
-
- Kasid U, Dritschilo A. RAF antisense oligonucleotide as a tumor radiosensitizer. Oncogene. 2003;22:5876–5884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

