Neonatal Histamine-2 Receptor Antagonist and Proton Pump Inhibitor Treatment at United States Children's Hospitals
- PMID: 27131401
- PMCID: PMC4925209
- DOI: 10.1016/j.jpeds.2016.03.059
Neonatal Histamine-2 Receptor Antagonist and Proton Pump Inhibitor Treatment at United States Children's Hospitals
Abstract
Objective: To determine treatment frequency and duration of histamine-2 receptor antagonist (H2RA)/proton pump inhibitor (PPI) use among infants hospitalized within US children's hospital neonatal intensive care units and evaluate diagnoses/demographic factors associated with use.
Study design: We retrospectively analyzed a cohort of neonatal intensive care unit infants admitted to 43 US children's hospitals within the Pediatric Health Information System database between January 2006 and March 2013 to determine H2RA/PPI treatment frequency, timing/duration of treatment, factors associated with use, percent of infants remaining on treatment at discharge, and interhospital prescribing variation. We used a modified Poisson regression to calculate the adjusted probability of infants ever receiving H2RAs/PPIs in relation to diagnosis, gestation, and sex.
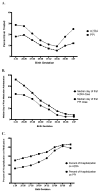
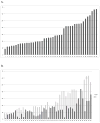
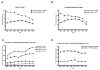
Results: Of the 122 002 infants evaluated, 23.8% (n = 28 989) ever received an H2RA or PPI; 19.0% received H2RAs (n = 23 187), and 10.5% (n = 12 823) received PPIs. Extremely preterm infants and term infants were the most likely to receive H2RA and PPI treatment. Infants with gastroesophageal reflux disease (relative risk [RR] = 3.13) and congenital heart disease (RR = 2.41) had the highest H2RA/PPI treatment probabilities followed by those with an ear, nose, and throat diagnosis (RR = 2.34; P < .05). The majority of treated infants remained treated at discharge.
Conclusions: Despite limited evidence and increasing safety concerns, H2RAs/PPIs are frequently prescribed to extremely preterm neonates and those with congenital anomalies and continued through discharge. Our findings support the need for innovative studies to examine the comparative effectiveness and safety of H2RA/PPIs vs no treatment in these high-risk neonatal populations.
Keywords: H2-receptor antagonist; comparative effectiveness; drug utilization; gastroesophageal reflux disease; neonatal; patient-centered outcomes; pharmacoepidemiology; practice variation; proton pump inhibitor; stress ulcer prophylaxis.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–87. - PubMed
-
- Malcolm WF, Gantz M, Martin RJ, Goldstein RF, Goldberg RN, Cotten CM. Use of medications for gastroesophageal reflux at discharge among extremely low birth weight infants. Pediatrics. 2008;121:22–7. - PubMed
-
- Jadcherla SR. Gastroesophageal reflux in the neonate. Clin Perinatol. 2002;29:135–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

